Suppression method of radionuclide deposition on reactor component of nuclear power plant and ferrite film formation apparatus
a technology of ferrite film and radionuclide, which is applied in the direction of nuclear engineering, nuclear elements, greenhouse gas reduction, etc., to achieve the effect of suppressing radionuclide deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
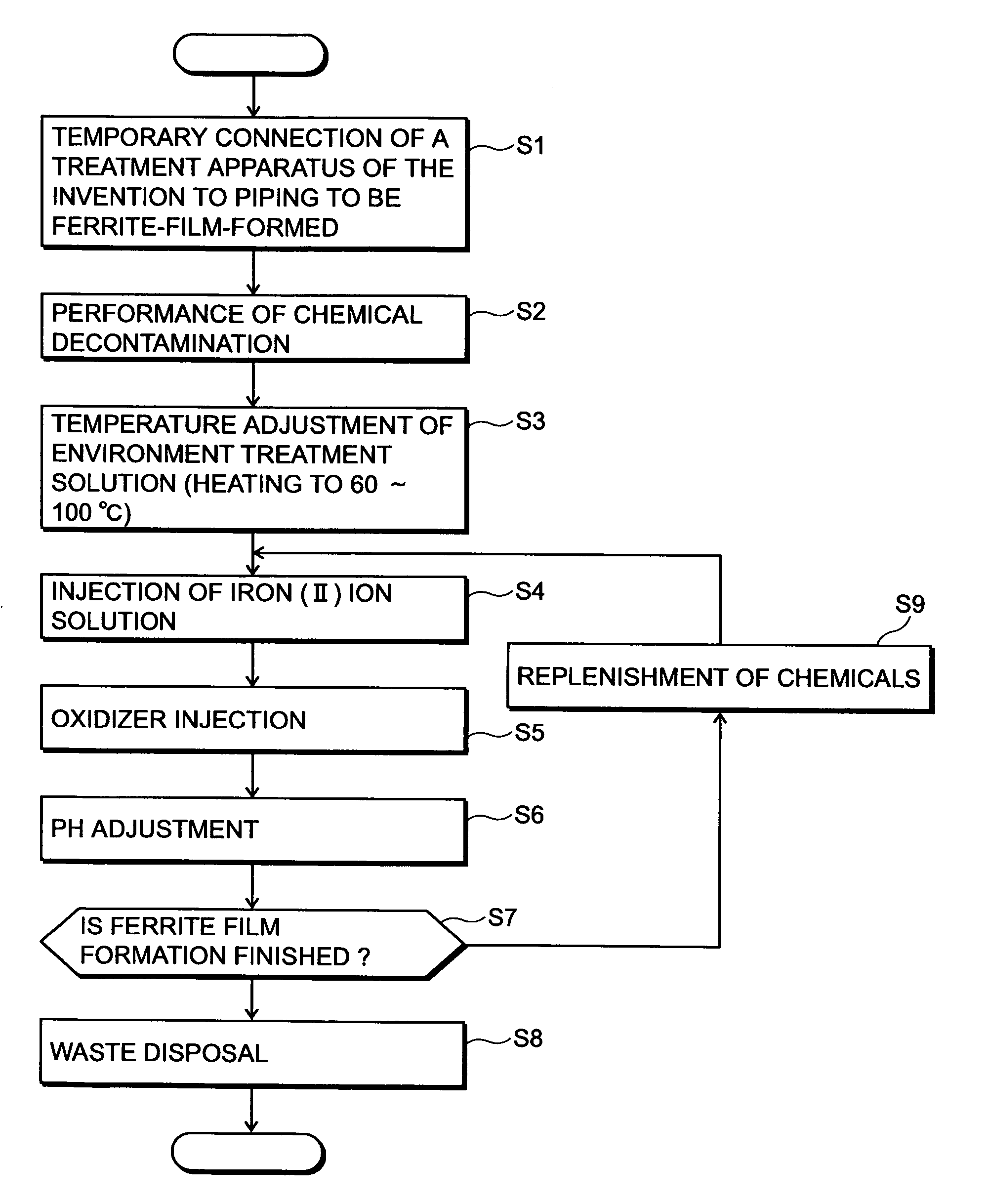
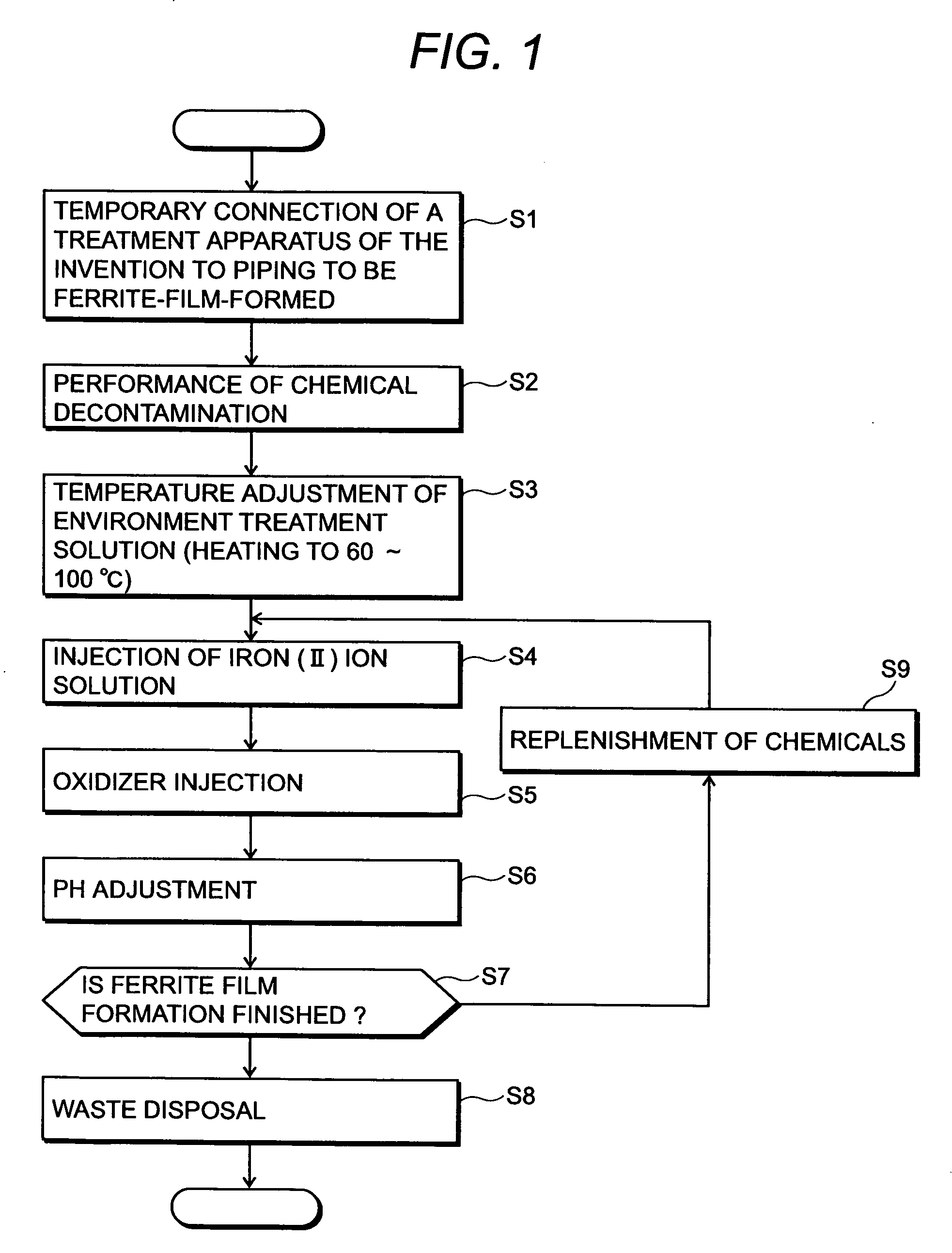
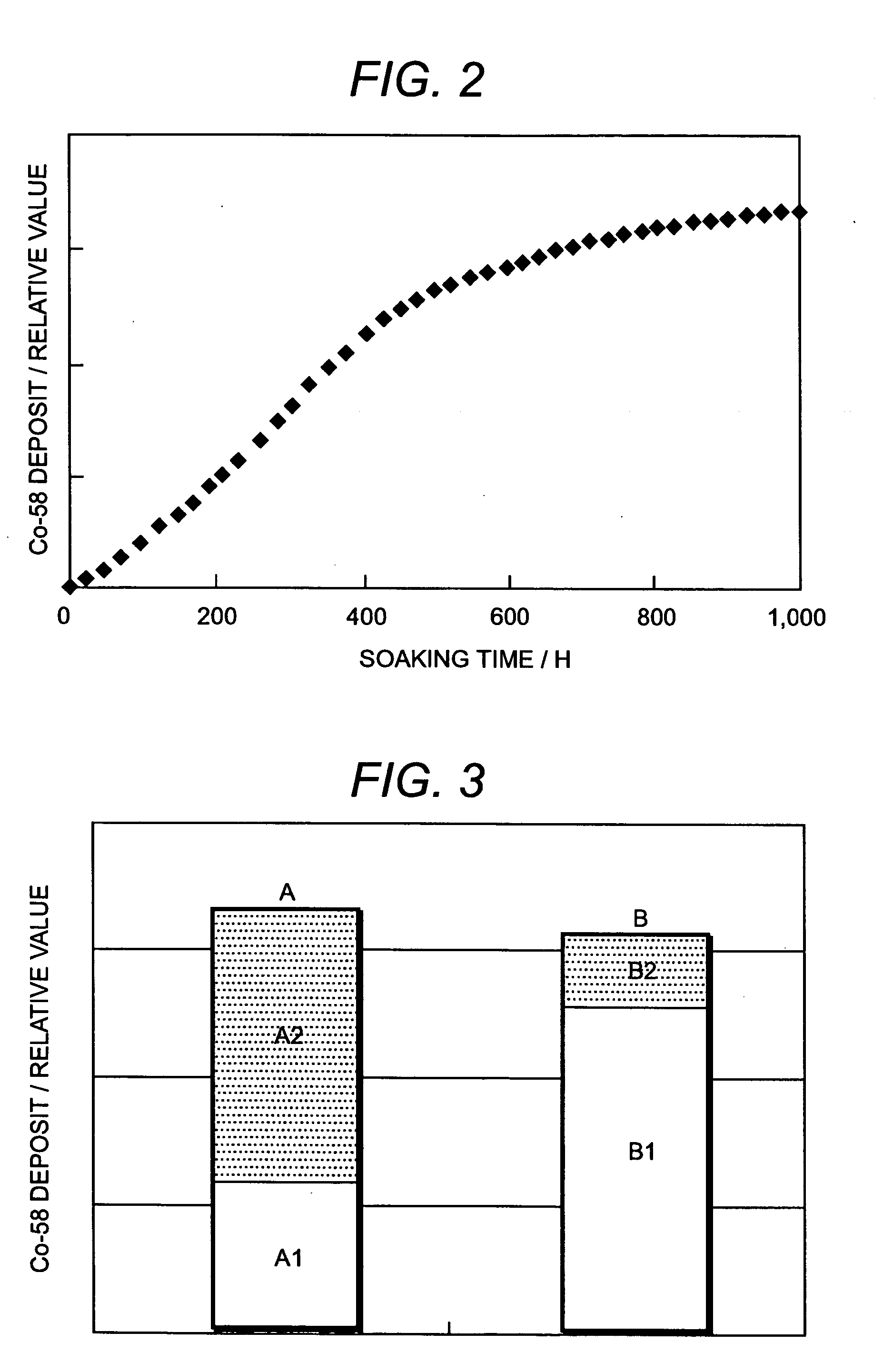
[0050]FIG. 1 shows a flowchart of an embodiment of a radionuclide deposition suppression method of the present invention, FIG. 5 shows a schematic diagram of a whole system construction of an embodiment in which the present invention is applied to recirculation piping of a nuclear power plant, and FIG. 6 shows a schematic diagram of a detailed system construction of a film forming apparatus for practicing the radionuclide deposition suppression method according to the present invention.
[0051] As shown in FIG. 5, the nuclear power plant comprises a nuclear reactor 1 having fuel rods contained in a pressure vessel, main steam piping 2 connected to the top of the nuclear reactor 1, a steam turbine generator 3 connected to the main steam piping 2, and a condenser 4 connected to a steam outlet of the steam turbine generator 3. The condensate condensed in the condenser 4 is taken out by a condensate pump 5, and returned as feed water for the nuclear reactor 1 through a feed water piping ...
second embodiment
[0068]FIG. 8 shows a system of a concrete embodiment of a portion forming iron (II) ions stored in the chemical tank 49 shown in FIG. 6. In FIG. 8, formic acid solution is stored in a chemical tank 70, and nitrogen from nitrogen bubbling equipment 71 is bubbled in the chemical tank 70, whereby dissolved oxygen is removed. The formic acid from which the dissolved oxygen is removed is transferred to a metal iron dissolution tank 73 by an injection pump 72. Metal iron 74 is set in the metal iron dissolution tank 73, and iron (II) ion is dissolved by the formic acid transferred thereto. At this time, nitrogen from the nitrogen bubbling equipment 71 is bubbled in the metal iron dissolution tank 73. The formic acid solution in the metal iron dissolution tank 73 does not substantially include dissolved oxygen, so that the iron (II) ion is almost not oxidized to iron (III) ion. The formic acid solution which dissolved the iron (II) ion is transferred to the chemical tank 49 by an injection ...
third embodiment
[0070]FIG. 9 shows a system of another embodiment of iron (II) ion formation shown in FIG. 8. In the present embodiment, carbonic water instead of formic acid in the embodiment of FIG. 8 is used as an iron ion solution. Carbonic acid is stored in a chemical tank 76, carbon dioxide supplied form a carbon dioxide bubbling equipment 77 is bubbled into the chemical tank 76, and saturated carbonic water of one atmosphere is formed. The formed one atmosphere saturated carbonic water is transferred to the metal iron dissolution tank 73 by the injection pump 72. Iron (II) carbonate 78 is contained in the metal iron dissolution tank 73, and the one atmosphere saturated carbonic water is dissolved. Further, carbon dioxide from the carbon dioxide bubbling equipment 71 is bubbled into the metal ion dissolution tank 73. By the way, according to the literature (Chemical Unabridged Dictionary 5, page 729 (1997), Reduced-size edition), usually, the solubility of iron (II) carbonate is 0.065 g / 100 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com