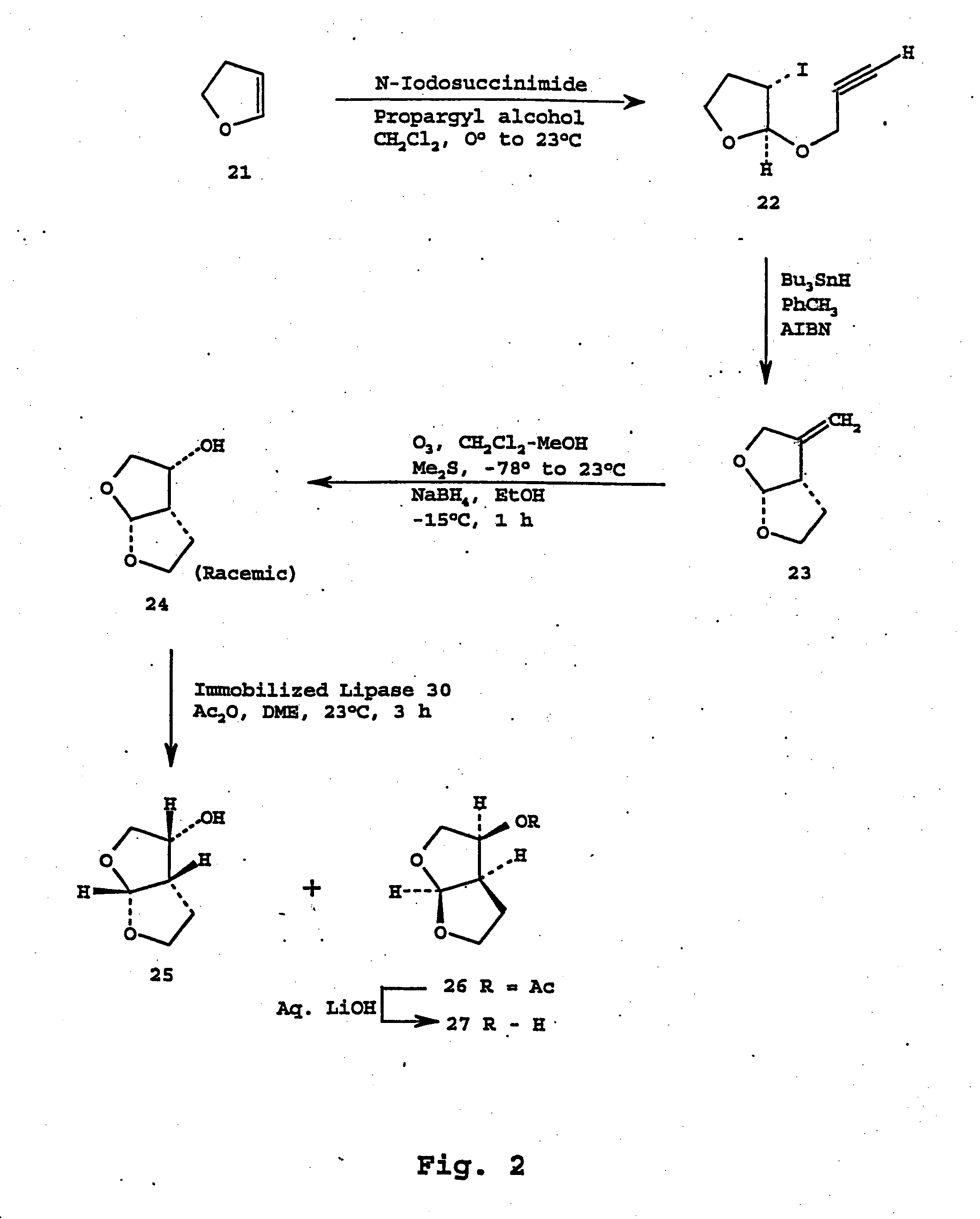
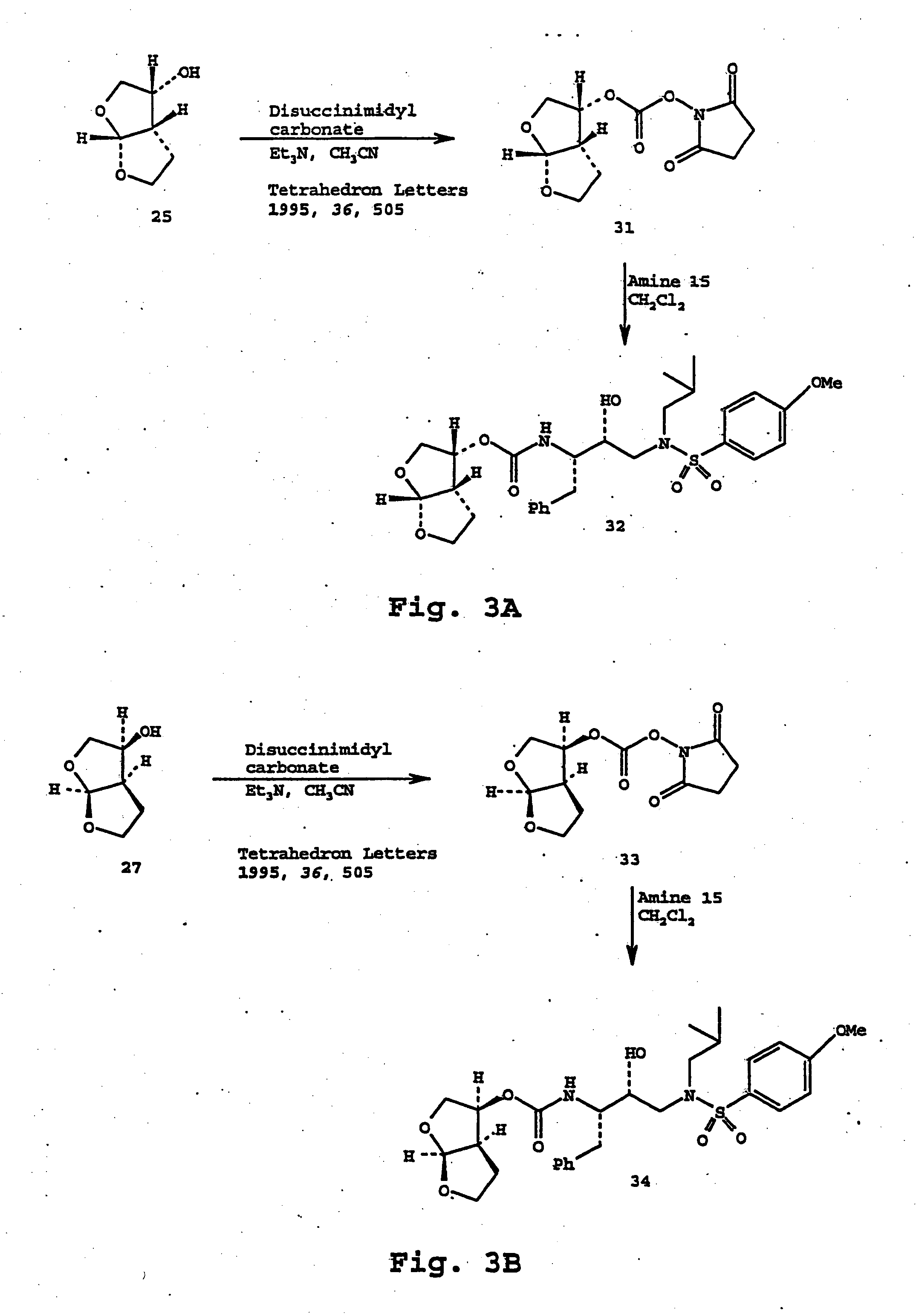
Fitness assay and associated methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
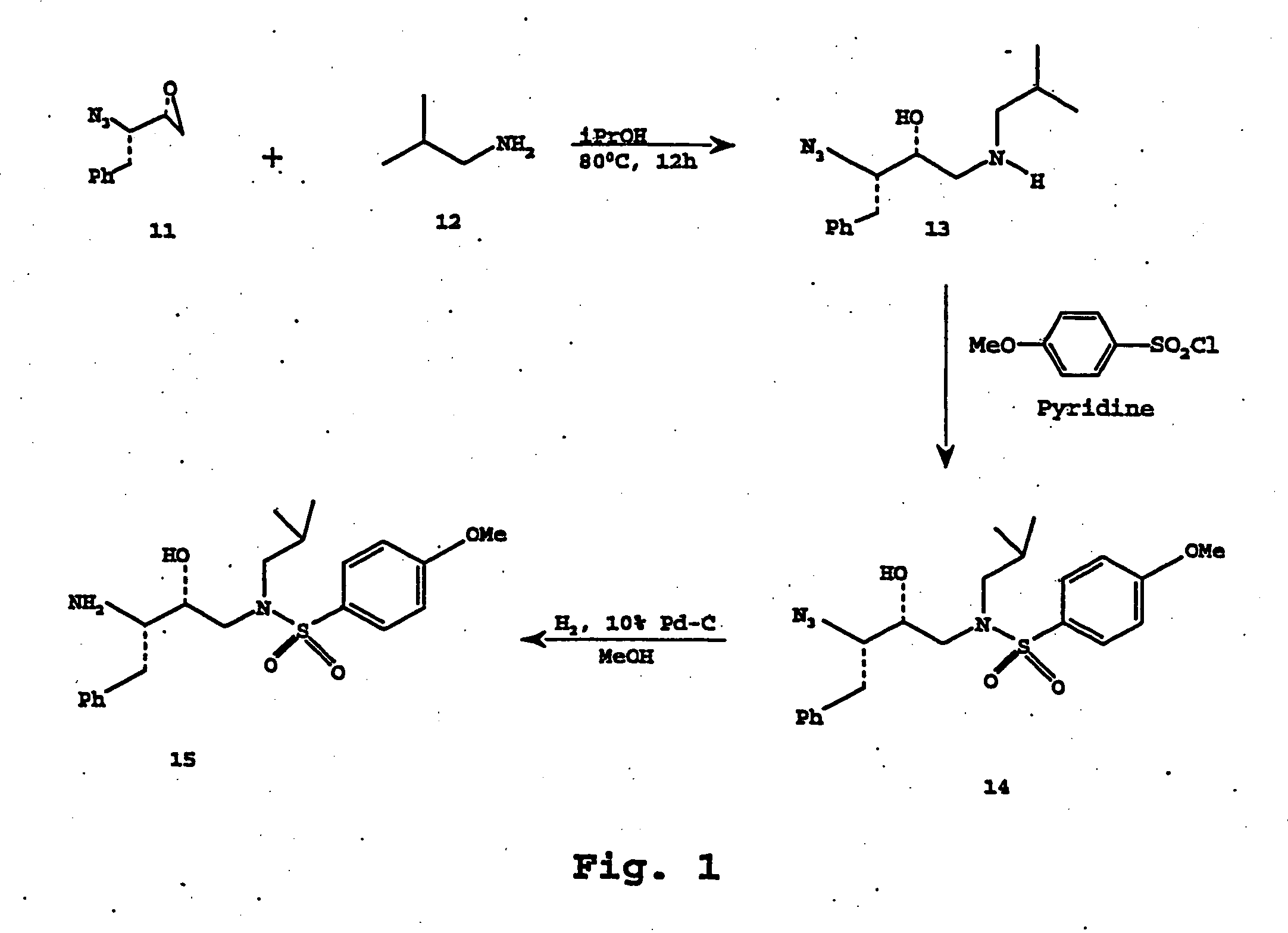
[0190] This example describes the synthesis of exemplary epoxide 11 (FIG. 1), which is used as an intermediate in the synthesis of a particular series of compounds within the scope of the present invention.
[0191] Anhydrous CuCN (4.86 g, 54 mmol) was added to a solution of butadiene monooxide (38 g, 540 mmol) in anhydrous tetrahydrofuran (1.2 L) and the resulting mixture was stirred at −78° C. Commercial phenyl magnesium bromide solution (Aldrich) in ether (65 mmol) was added dropwise over a period of 10 min. The resulting reaction mixture was then allowed to warm to 0° C. and it was continued to stir until the reaction mixture was homogeneous. After this period, the reaction mixture was cooled to −78° C. and 0.58 mole of phenylmagnesium bromide solution in ether was added dropwise for 30 min. The reaction mixture was allowed to warm to 23° C. for 1 h. The reaction was quenched by slow addition of saturated aqueous NH4Cl (120 mL) followed by NH4OH (70 mL), saturated NH4Cl (500 ML) a...
example 2
[0196] This example illustrates the synthesis of azidoalcohol 13 (FIG. 1), which can be used as an intermediate in the synthesis of a preferred series of the compounds of the present invention.
[0197] To a stirred solution of above azidoepoxide 11 (700 mg, 3.7 mmol) in ispropanol (70 mL) was added isobutyl amine (Aldrich, 0.74 mL 7.4 mmol) and the resulting mixture was heated at 80° C. for 12 h. After this period, the reaction mixture was concentrated under reduced pressure and the residue was chromatographed over silica gel to provide azidoalcohol 13 (800 mg) as an oil.
example 3
[0198] This example illustrates the synthesis of azidosulfonamide 14, the structure of which is shown in FIG. 1.
[0199] To a stirred solution of 13 (600 mg, 2.28 mmol) in CH2Cl2 (20 mL) was added 4-methoxybenzenesulfonyl chloride (Aldrich, 530 mg, 2.52 mmol) and saturated aqueous NaHCO3 (6 mL). The resulting heterogeneous mixture was stirred at 23° C. for 12 h. The reaction was diluted with CH2Cl2 and the layers were separated. The organic layer was washed with brine, dried over anhydrous magnesium sulfate and concentrated to dryness. The residue was chromatographed over silica gel (25% ethyl acetate / hexane) to provide 900 mg of azidosulfonamide 14.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com