Enzyme reaction method and a method for enzymatically producing an optically active cyanohydrin
a technology of cyanohydrin and reaction method, which is applied in the direction of lyase, fermentation, etc., can solve the problems of extended reaction time, inability to carry out such a reaction in an aqueous system, and often denatured enzymolysis and thus generally unstable in an organic solvent system, etc., and achieve the effect of sufficient reaction ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
Preparation of (R)-hydroxynitrile Lyase
[0145] (1) 100 g of ground almond (Prunus amygdalus) seeds was mixed and stirred with 200 ml acetone for 2 hours, and then filtrated to collect the solids. After drying, the solids were mixed with 600 g of water and adjusted to pH 7.5 with aqueous ammonia, followed by overnight mixing with stirring. Subsequently, the resulting slurry was centrifuged to collect the supernatant, which was then adjusted to pH 5.5 and centrifuged to give a solution free from insoluble components.
[0146] (2) The (R)-hydroxynitrile lyase enzyme solution prepared in (1) above was assayed for its activity. The production rate of benzaldehyde decomposed from DL-mandelonitrile (substrate) by the action of the enzyme was measured as a change in absorbance at 249.6 nm to calculate enzyme activity. An enzyme activity at which 1 μmol of benzaldehyde is produced per minute is defined as 1 unit (U). The enzyme solution prepared in (1) above was assayed in this manner, indicat...
preparation example 2
Preparation of (S)-hydroxynitrile Lyase
[0147] (1) (S)-hydroxynitrile lyase was prepared using yeast, Saccharomyces cerevisiae, as a host by introducing the (S)-hydroxynitrile lyase gene cloned from Manihot esculenta into the yeast and then culturing the resulting recombinant yeast. The recombinant yeast was cultured in 1 L of YPD medium containing 1% yeast extract, 2% peptone and 2% glucose for 24 hours to collect the yeast cells, which were then homogenized and purified to give a solution free from insoluble components.
[0148] (2) The (S)-hydroxynitrile lyase enzyme solution prepared in (1) above was assayed in the same manner as described in Preparation Example 1 (2), indicating that 9,000 units of enzyme having an activity of 40 U / ml can be collected.
preparation example 3
Preparation of Immobilized (R)-hydroxynitrile Lyase
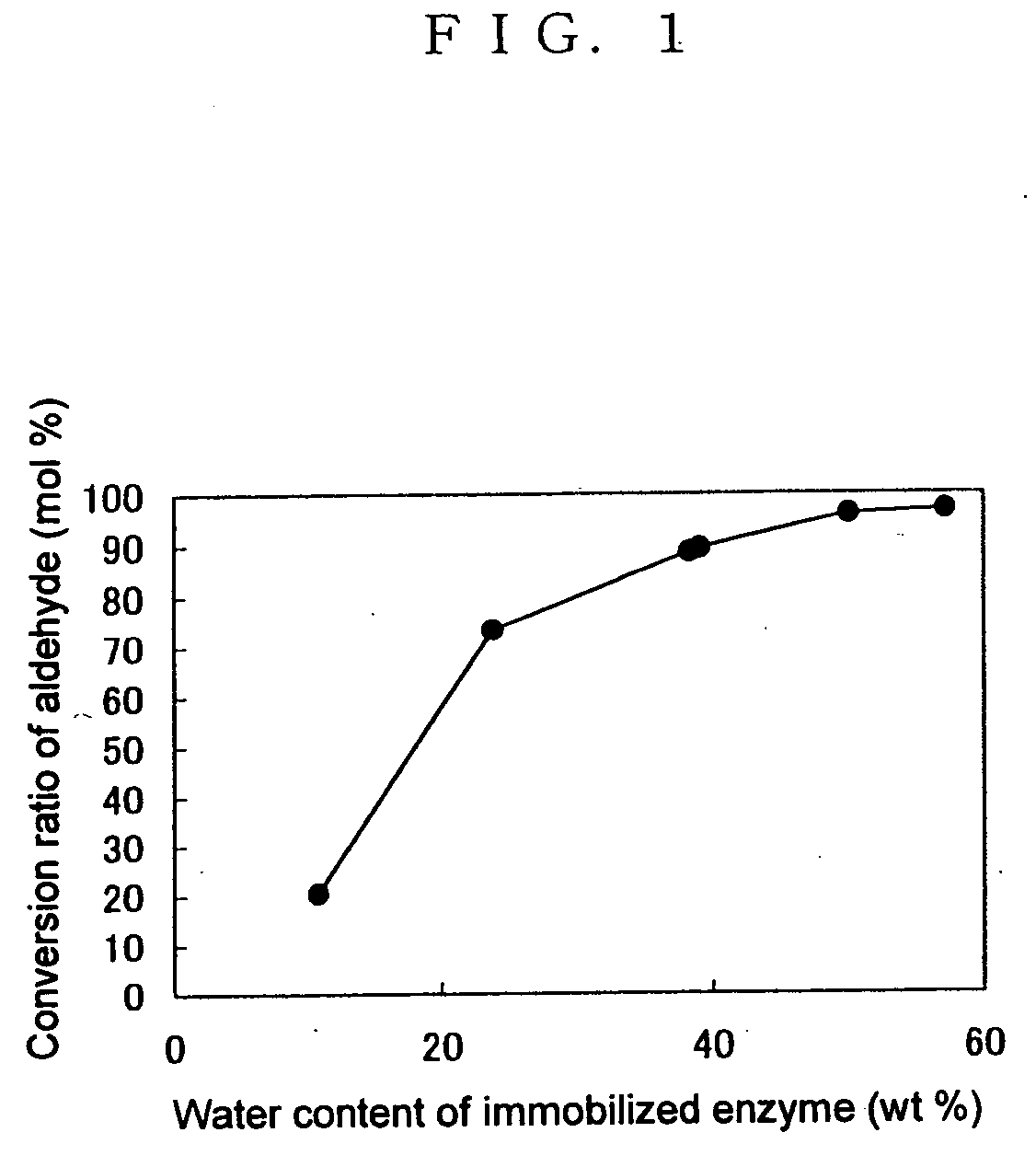
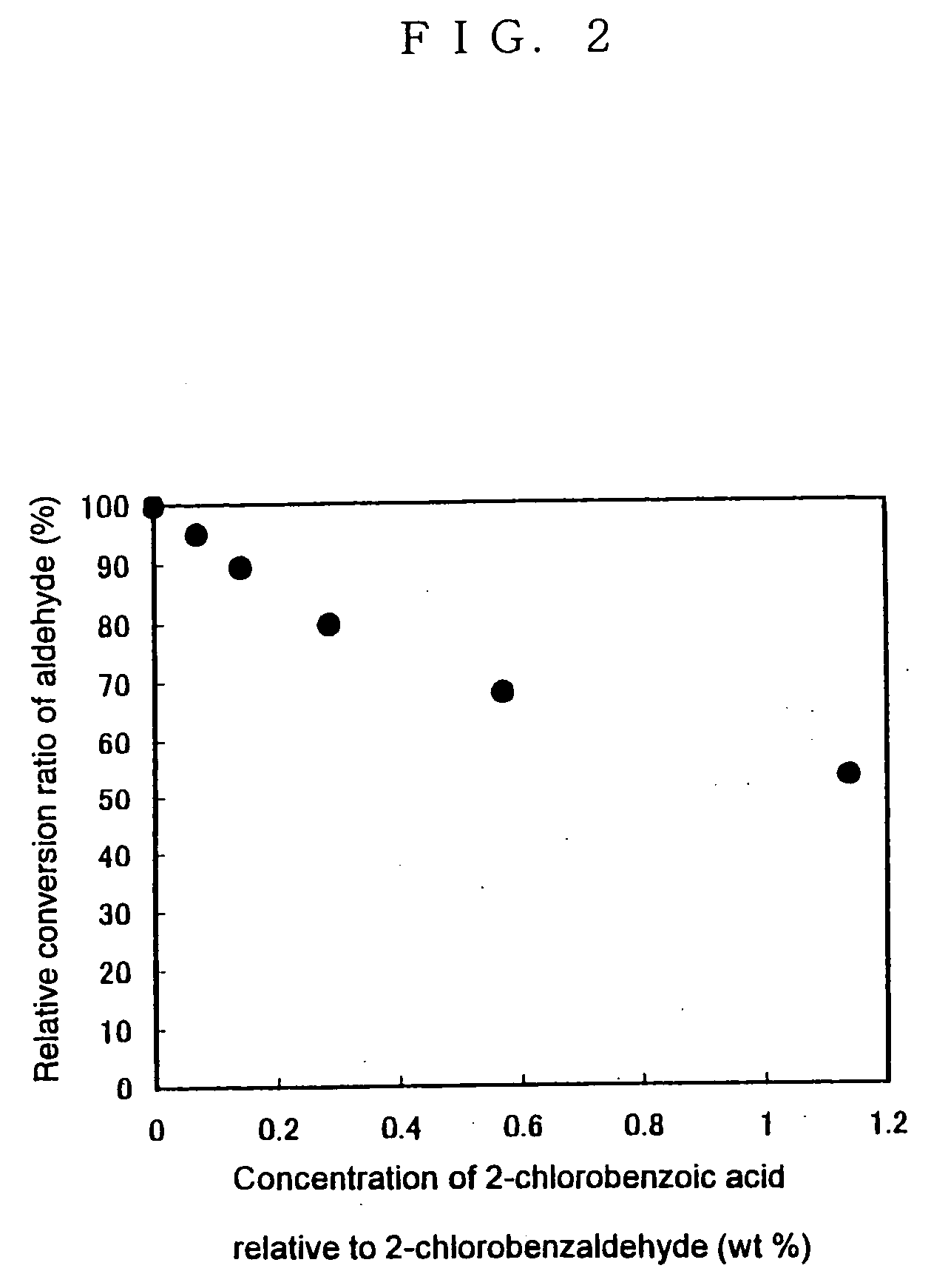
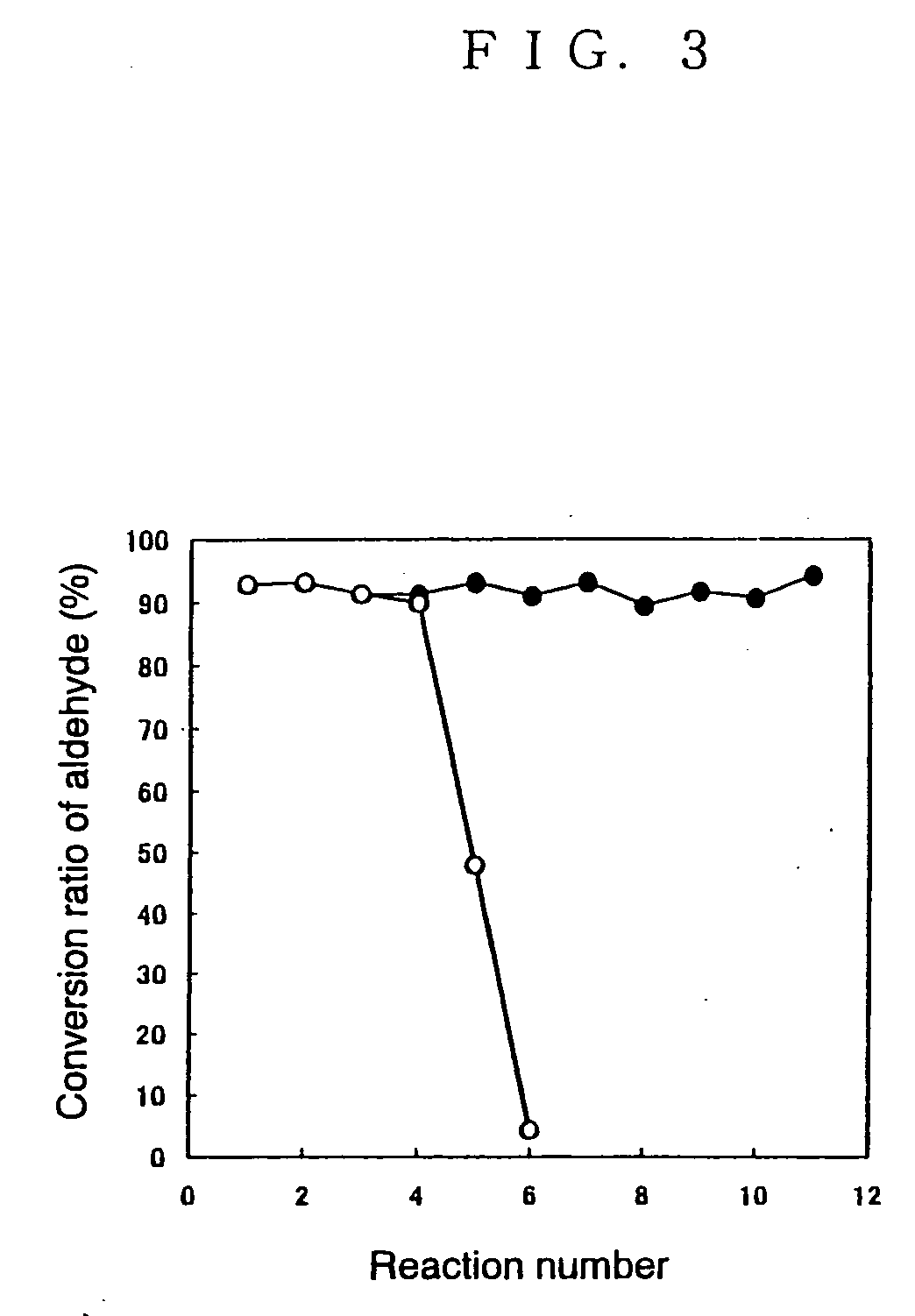
[0149] The (R)-hydroxynitrile lyase enzyme solution prepared in Preparation Example 1 was subjected to ammonium sulfate precipitation to concentrate the enzyme, thereby providing a 1000 U / ml enzyme solution. Carriers for immobilization (porous silica gel; microbead silicagel 300A, Fuji Silysia Chemical Ltd.) were mixed with this enzyme solution in an amount of 1 g per ml of enzyme solution. This mixture was used as such for the synthesis reaction. This immobilized enzyme had a water content of 50% by weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com