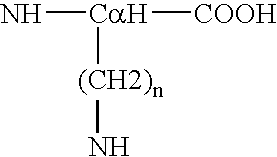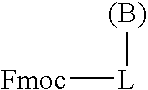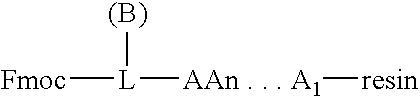Process for the determination of peptides corresponding to immunologically important epitopes and their use in a process for determination of antibodies or biotinylated peptides corresponding to immunologically important epitopes, a process for preparing them and compositions containing them
a technology of immunologically important epitopes and peptides, which is applied in the field of process for the determination of peptides corresponding to immunologically important epitopes, can solve the problems of not being able to obtain antibodies, not possessing the correct overall charge or amino acid composition, and not being able to identify antibodies to molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Synthesis of N-.alpha.-Fmoc-Lys (N-.epsilon.-biotin)
[0324] A. Method A
[0325] Commercially available N-.alpha.-Fmoc-L-lysine (N-.epsilon.-tBoc) (1.5 grams) was treated with 20 milliliters of 95% trifluoroacetic acid, 5% H.sub.2O for 2 hours at room temperature. Most of the acid was then evaporated under a stream of nitrogen. Ten milliliters of water was added and the solution was extracted 3 times with diethylether. The aqueous phase was then evaporated to dryness in vacuo over phosphorus pentoxide. The resulting powder (N-.alpha.-Fmoc-L-lysine) was analyzed by reverse phase chromatography and revealed a homogeneous product which was, as expected, more hydrophilic than the starting material.
[0326] N-.alpha.-Fmoc-lysine (190 mg, 0.49 mmol) was dissolved in 8 milliliters of 0.1 M borate buffer, pH 8.7. N-hydroxysuccinimidobiotin (162 mg, 0.47 mmol) was dissolved in 4 milliliters of dimethylformamide and added to the solution of N-.alpha.-Fmoc-lysine. The pH was monitored and titrated a...
example 3
Methods for the Determination of Peptides Corresponding to Immunologically Important Epitopes in an Enzyme-Linked Immunosorbent Assay (Elisa) Using Specific Antibodies
[0331] Where peptides were to be coated directly, stock solutions of the peptides were diluted in sodium carbonate buffer, pH 9.6 and used to coat polystyrene microtiter plates at a peptide concentration of 2 to 5 micrograms per milliliter for 1 hour at 37.degree. C.
[0332] In cases where biotinylated peptides were to be evaluated, plates were first coated with streptavidin in sodium carbonate buffer, pH 9.6 at a concentration of 3 micrograms per milliliter for 1 hour at 37.degree. C. The plates were then washed to remove excess, unbound protein. A working solution of the biotinylated peptide at 1 microgram per milliliter in sodium carbonate buffer was then added to the wells of the microtiter plate and incubated for 1 hours at 37.degree. C.
[0333] Once the plates had been coated with antigen, any remaining free binding ...
example 4
Use of Biotinylated HIV Peptides for the Detection of HIV-Specific Antibodies
[0336] Experiments were performed to evaluate antibody recognition of short, 10 amino acid-long, N-acetylated peptides corresponding to other contained within the transmembrane proteins of HIV-1 and HIV-2. Direct coating of these peptides in the wells of microtiter plates gave very poor results when antibody binding was evaluated in an ELISA. Since it was suspected that the peptides did not bind well to the polystyrene solid phase, the peptides were resynthesized in the same way except that biotin was attached to the amino terminus of the peptides, separated from the decamer peptide sequence by three glycine residues whose function it was to serve as a linker arm. The peptides used for the comparison were as follows:
34 TM-HIV-1: Ac-Ile-Trp-Gly-Cys-Ser-Gly-Lys- (SEQ ID NO:110) Leu-Ile-Cys-NH.sub.2 TM-HIV-1 Bio Bio-Gly-Gly-Gly-Ile-Trp-Gly-Cys- (SEQ ID NO:111) Ser-Gly-Lys-Leu-Ile-Cys-NH.sub.2 TM-HIV-2 Ac-Ser-T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| detection wavelength | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com