Mutant aspartate aminotransferase and its preparing process and application
A technology of aspartate transaminase and aspartate transaminase, which is applied in the field of aspartate transaminase, can solve the problems of reduced activity of dicarboxylate substrates and reduced transamination activity, and achieve low production cost, good effect, and high thermal efficiency. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Improvement of aspartate aminotransferase activity and substrate specificity
[0050] 1. Determine the mutation site: use a rational design method to connect the three-dimensional structure and the one-dimensional structure of Escherichia coli aspartate aminotransferase (eAspAT) through sequence analysis software (Clustal W) and structure analysis software (Rasmol), according to The comparison of the amino acid sequence of the enzyme and the aspartate aminotransferase of the thermophilic bacteria and the comparison of the substrate specificity of the two enzymes (the substrate specificity of the enzyme in Escherichia coli and thermophilic bacteria are also different) using the software DeepView to select mutations The position and the amino acid residue of replacement determine that the amino acid residue phe at the 338th position in the Escherichia coli aspartate aminotransferase sequence (SEQ IN No.2) is closely related to the activity and substrate specific...
Embodiment 2
[0078] Embodiment 2: the improvement of aspartate aminotransferase thermostability
[0079] 1. Using a rational design method, the three-dimensional structure and one-dimensional structure of Escherichia coli aspartate aminotransferase were linked by sequence analysis software (Clustal W) and structure analysis software (Rasmol), and the software DeepView was used to determine the aspartate aminotransferase of Escherichia coli. The 221st amino acid residue Leu in the acid transaminase sequence is closely related to the stability of the enzyme, and replacing the amino acid residue at this position with an Asn residue can improve the thermostability of the enzyme.
[0080] 2. The establishment of the mutant gene library includes the following steps:
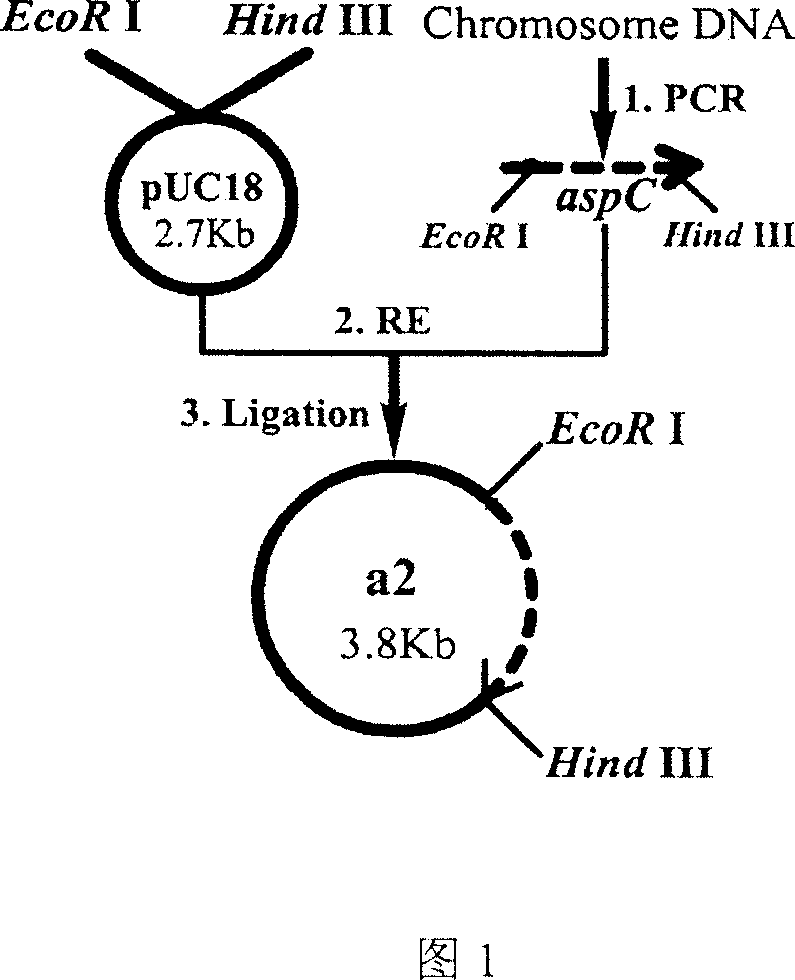
[0081] (1) Insert the Escherichia coli aspartate aminotransferase gene into pUC18 to construct a recombinant plasmid, and the corresponding genetically engineered bacterium is named A2, and the construction route is shown in FIG. 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Michaelis constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com