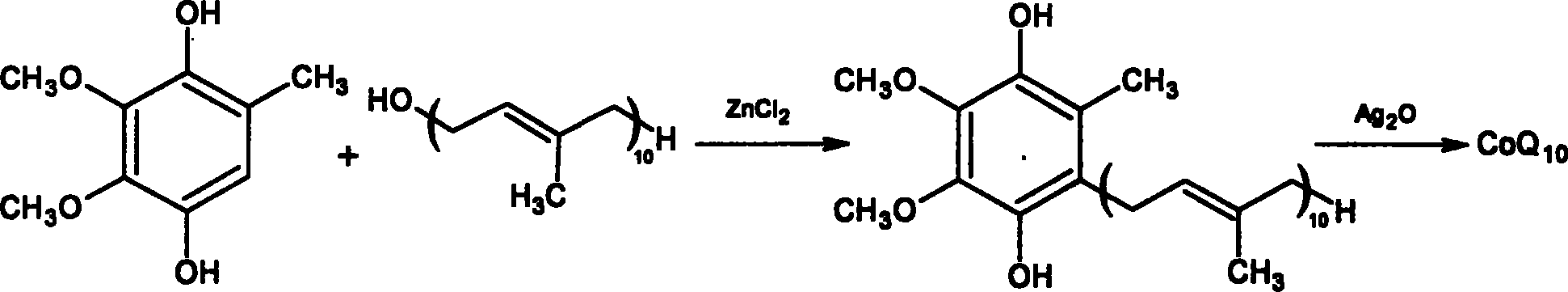
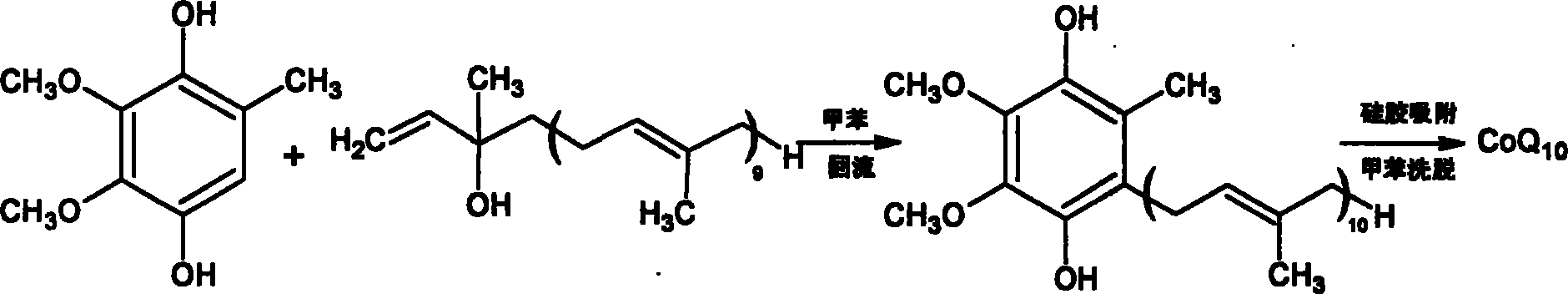
Coenzyme Q10 synthesizing process
A technology of coenzyme and hydroquinone, which is applied in the field of synthesizing coenzyme Q10, can solve the problems of unfavorable industrial production, harsh reaction conditions, and no yield, and achieve the effects of safe and controllable production process, mild reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
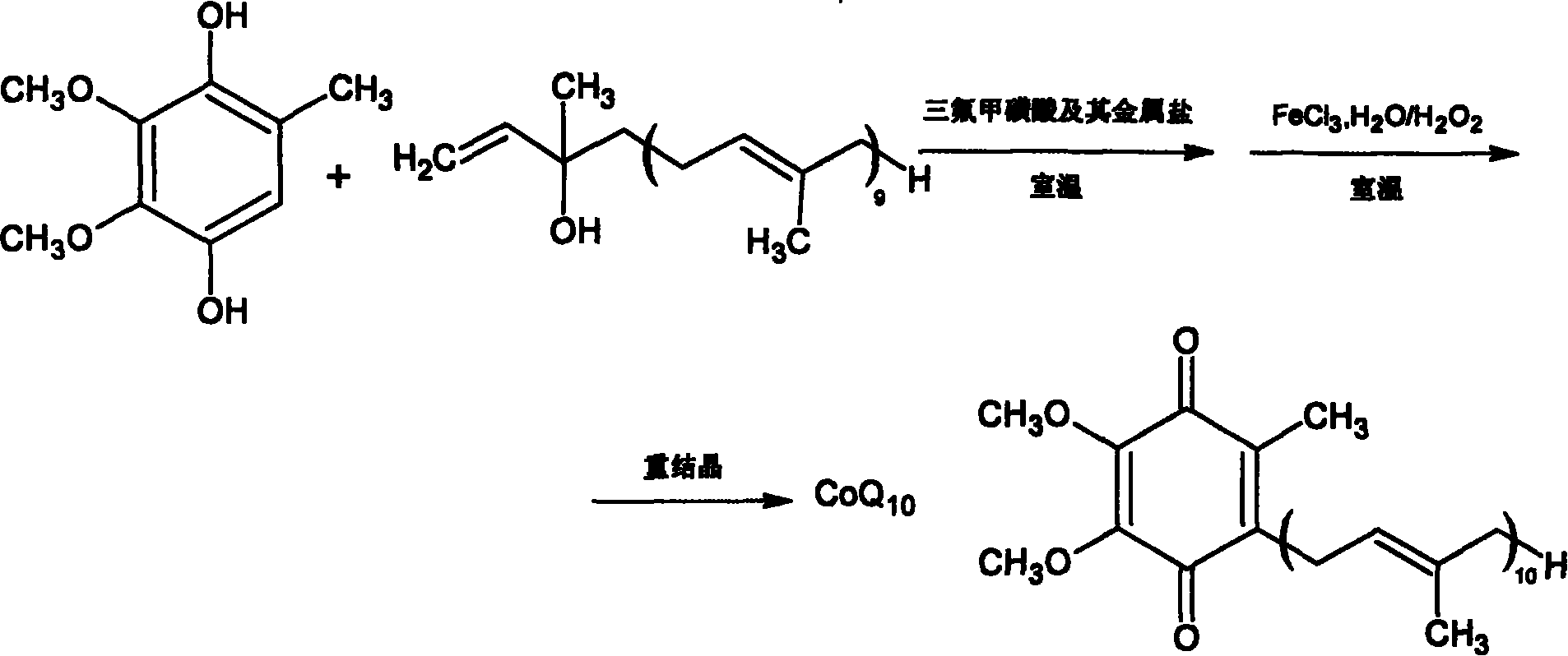
[0042] 30g coenzyme Q 0 Mix hydroquinone with 160ml of dry toluene, replace the air with nitrogen, heat and reflux in nitrogen atmosphere, and remove coenzyme Q through a water separator 0 Moisture contained in hydroquinone, finally evaporate toluene to dryness under reduced pressure, monitor moisture, coenzyme Q with moisture 0 Hydroquinone was stored under dry nitrogen for later use.
[0043] Mix 50g of isodecadecaprenyl alcohol with 330ml of toluene, and use a similar method to reflux under reduced pressure to separate and remove the moisture in the isodecadecaprenyl alcohol. Prenyl Alcohol is set aside.
Embodiment 2
[0045] Take the prepared coenzyme Q 0 Hydroquinone 3.68g (0.02mmol) and content are the isodecadecaprenyl alcohol 17.95g (0.025mmol) of 97.2%, join in the 100ml there-necked flask that is filled with nitrogen, add 12ml dichloromethane and 40ml of cyclopentane was stirred to dissolve all the raw materials, cerium trifluoromethanesulfonate with 1% isodecadecaprenyl alcohol was added to catalyze the reaction, the reaction was monitored by TLC, and the reaction was carried out at room temperature for 80 minutes. After the reaction is complete, add the reaction liquid into 50 ml of an oxidizing agent composed of hydrogen peroxide and ferric chloride solution, stir, monitor the reaction by TLC, and react at room temperature for 1 hour. Transfer to a separatory funnel for static layering, release the oxidant in the lower layer, wash the upper organic layer once with 20ml saturated brine, discard the brine layer, add 5g of anhydrous magnesium sulfate and dry for 2 hours, recover the s...
Embodiment 3
[0048] Take the dehydrated coenzyme Q 0 Hydroquinone 4.6g (0.025mmol) and content are 17.95g (0.025mmol) of isodecadecaprenyl alcohol of 97.2%, join in the 100ml there-necked bottle that is filled with nitrogen, add 8ml chloroform and 40ml n-hexyl in the bottle alkane, stirring to dissolve all the raw materials, adding hafnium trifluoromethanesulfonate with a molar weight of 3% isodecadecaprenyl alcohol to catalyze the reaction, monitoring the reaction by TLC, and reacting at room temperature for 120 minutes. After the reaction is complete, add the reaction solution into 60 ml of an oxidizing agent composed of hydrogen peroxide and ferric chloride solution, stir, monitor the reaction by TLC, and react at room temperature for 1.5 hours. Transfer to a separatory funnel for static layering, release the oxidant in the lower layer, wash the upper organic layer once with 20ml of saturated brine, discard the brine layer, add 5g of anhydrous magnesium sulfate to dry for 2 hours, recov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com