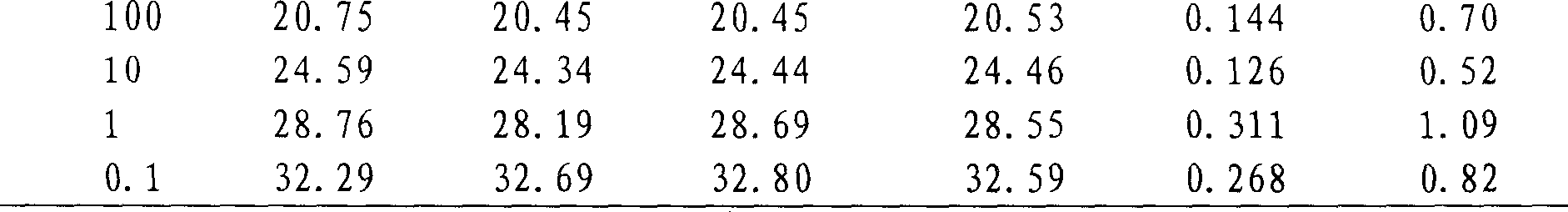Measles virus fluorescent augmentation detection kit and detection method
A detection kit and technology for measles virus, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of high false positive rate and easy contamination in detection, achieve high sensitivity, convenient and accurate judgment, and achieve quantitative The effect of analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 11 patient gargles (collection time is 3 to 4 days after the rash) were applied to the TaqMan fluorescent quantitative RT-PCR method of the present invention for 2 outbreaks of measles, and the conventional RT-PCR method and virus isolation method were used to detect, wherein the virus 2 were isolated positive, 7 were positive by RT-PCR, and 10 were positive by TaqMan RT-PCR. The positive results of these three tests were consistent. 15 days after the rash appeared, the serum measles virus IgM antibody of the patients was measured. Among the 11 patients, 10 cases of measles IgM antibody were positive, which was completely consistent with the results of TaqMan RT-PCR.
[0060] The TaqMan RT-PCR reaction system is as follows:
[0061] 2×RT-PCR buffer 12.5μL
[0062] RNase inhibitor RNasin 0.8 activity unit / μL
[0063] Specific amplification upstream primer 0.4μM
[0064] Specific amplification downstream primer 0.4μM
[0065] Specific probe 0.2μM
[0066] The deoxynu...
Embodiment 2
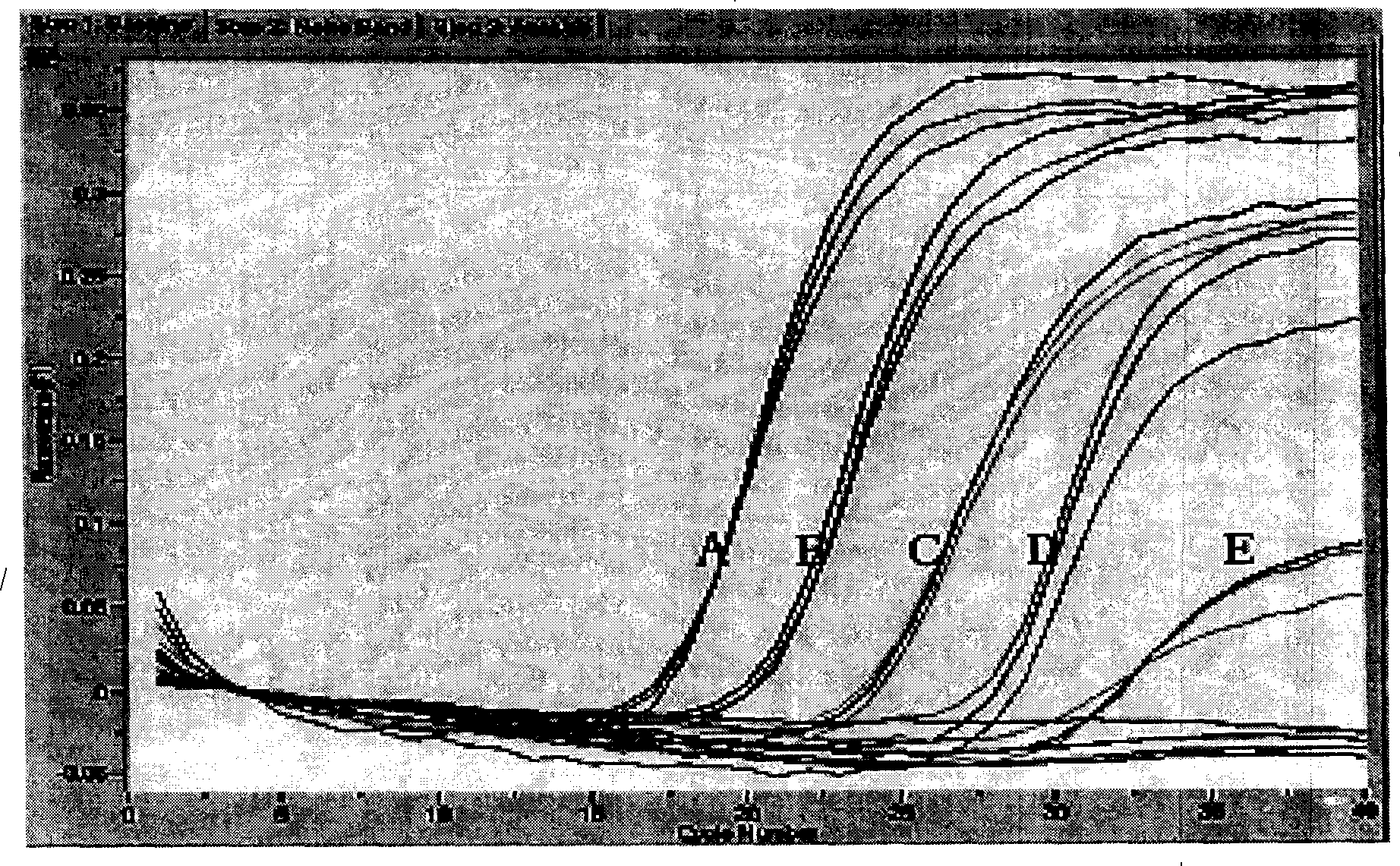
[0074] The measles virus strain Shanghai 191 with known virus titer was serially diluted by 10 times to 1000, 100, 10, 1, 0.1 TCID 50 5 gradients, each gradient takes 200 μL, extracts viral RNA, and detects by fluorescent quantitative PCR (the method is the same as in Example 1). For each concentration of the sample, it is repeated 3 times, and the results are shown in figure 1 . The composition of the fluorescent quantitative PCR reaction system is the same as in Example 1. The results show that the sensitivity of measles virus quantitative RT-PCR is 0.1TCID 50 .
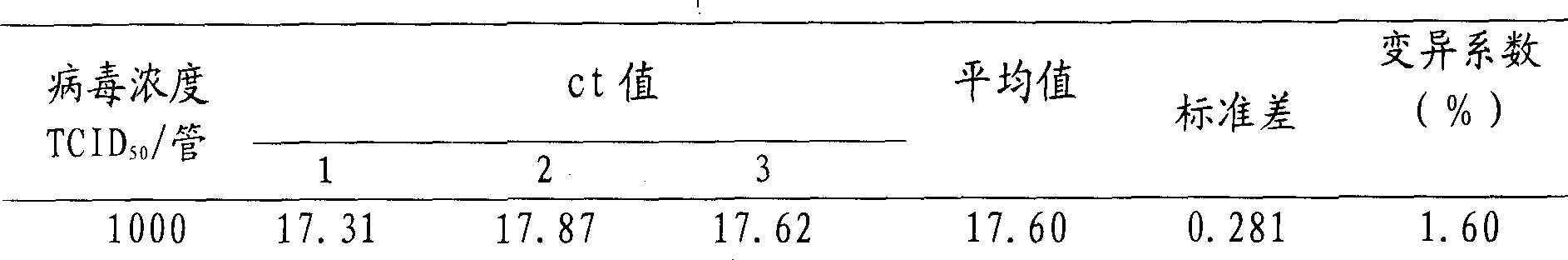
[0075] The repeatability of the method was evaluated by evaluating the coefficient of variation (CV) of the Ct value of three repeated experiments for each dilution, and the results are shown in Table 1. The coefficients of variation are all less than 3%, indicating that the method has good repeatability.
[0076] Table 1 Reproducibility of measles virus detection by fluorescent RT-PCR
[0077]
[0078] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com