Gene detection reagent kit for SARS virus and its detection method
A SARS virus and genetic detection technology, applied in the field of genetic detection kits, can solve problems such as unfavorable epidemic control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] The genetic detection kit of preferred SARS virus, comprises the partial sequence of SARS virus-specific nucleic acid quantitative standard substance namely SARS:
[0114] 5'-TACCGTAGACTCAATCTCTATGATGGGTTTCAAAATGAATTACCAAGTCA
[0115] ATGGTTACCCTAATATGTTTATCACCCGCGAAGAAGCTATTCGTCACGTTCG
[0116] TGCGTGGATTGGCTTTGATGTAGAGGGCTGTCATGCAACTAGAGATGCTGT
[0117] GGGTACTAACCTACCTCTCCAGCTAGGATTTTCTACAGGTGTTAACTTAGTA
[0118] GCTGTACCGACTGGTTATGTTGACACTGAAAATAACCAGAATTCACCAGA
[0119] GTTAATGCAAAACCTCCACCAGGTGACCAGTTTAAACATCTTATACC-3' and
[0120] (1) Fluorescence amplification detection reagent: It is made by mixing the following components with distilled water as solvent:
[0121] The one-step RT-PCR buffer is:
[0122] 50mM potassium chloride (KCl), 10mM Tris-Cl, 25mM magnesium chloride (MgCl2), 0.1% g / ml polyethylene glycol 6000, 0.1% 1,4-dimercaptothreitol (DTT), 1% g / ml Bovine serum albumin (BSA) deoxynucleoside triphosphate (dNTP mixture) is:
[0123] 200 μM dATP, 2...
Embodiment 2
[0150] The gene detection kit of preferred SARS virus, comprises SARS virus-specific nucleic acid quantitative standard as embodiment 1, also includes:
[0151] (1) Fluorescence amplification detection reagent: It is made by mixing the following components with distilled water as solvent:
[0152] The one-step RT-PCR buffer is:
[0153] 500mM potassium chloride (KCl), 100mM Tris-Cl, 25mM magnesium chloride (MgCl 2 ), 0.1% g / ml polyethylene glycol 6000, 0.1% 1,4-dimercaptothreitol (DTT), 1% g / ml bovine serum albumin (BSA). Deoxynucleoside triphosphate (dNTP mixture) is: dATP:dCTP:dGTP:dTTP:dUTP=4:4:4:4:1; the concentration used is 100μM
[0154] Specific primer S1 0.45μM
[0155] Specific primer S2 0.55μM
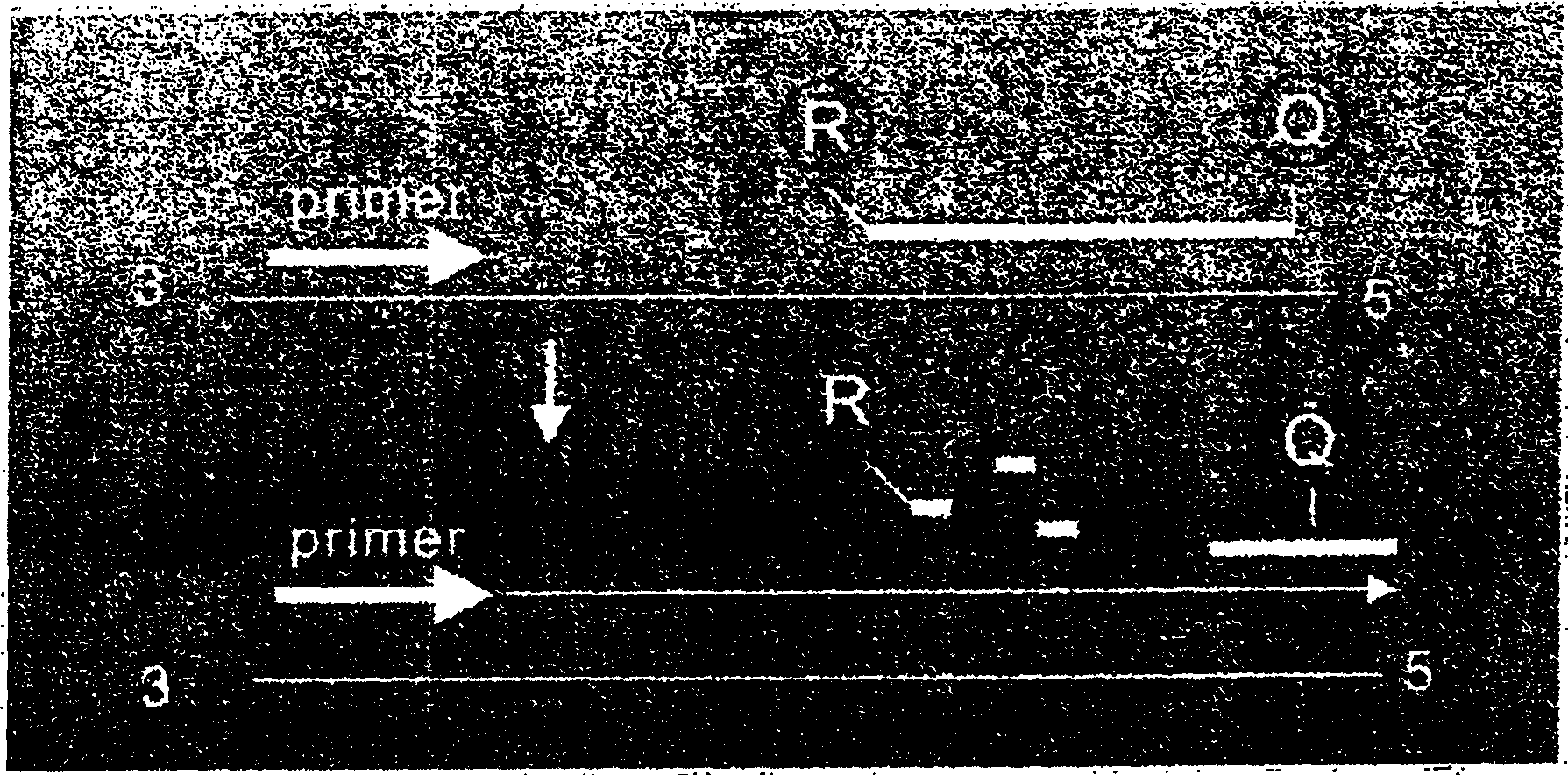
[0156] Specific probe S3 0.2μM, R is FAM (carboxy fluorescent yellow), Q is TAMARA (tetramethyl-6 carboxyrhodamine)
[0157] (2) DNA polymerase (Taq): select Ampli TaqR DNase, 1.5 enzyme activity units / reaction (U)
Embodiment 3
[0169] The gene kit of preferred SARS virus of the present invention is compared with the similar reagent that German ARTUS company produces:
[0170] Sample No. 1 and No. 7 are blank controls;
[0171] Sample No. 2~6 selects the kit and detection method of embodiment 1 to detect the SARS standard product result of different dilution gradients;
[0172] Samples 8 to 12 were tested with SARS virus gene detection reagents from ARTUS company in Germany to detect the results of SARS standard products with different dilution gradients.
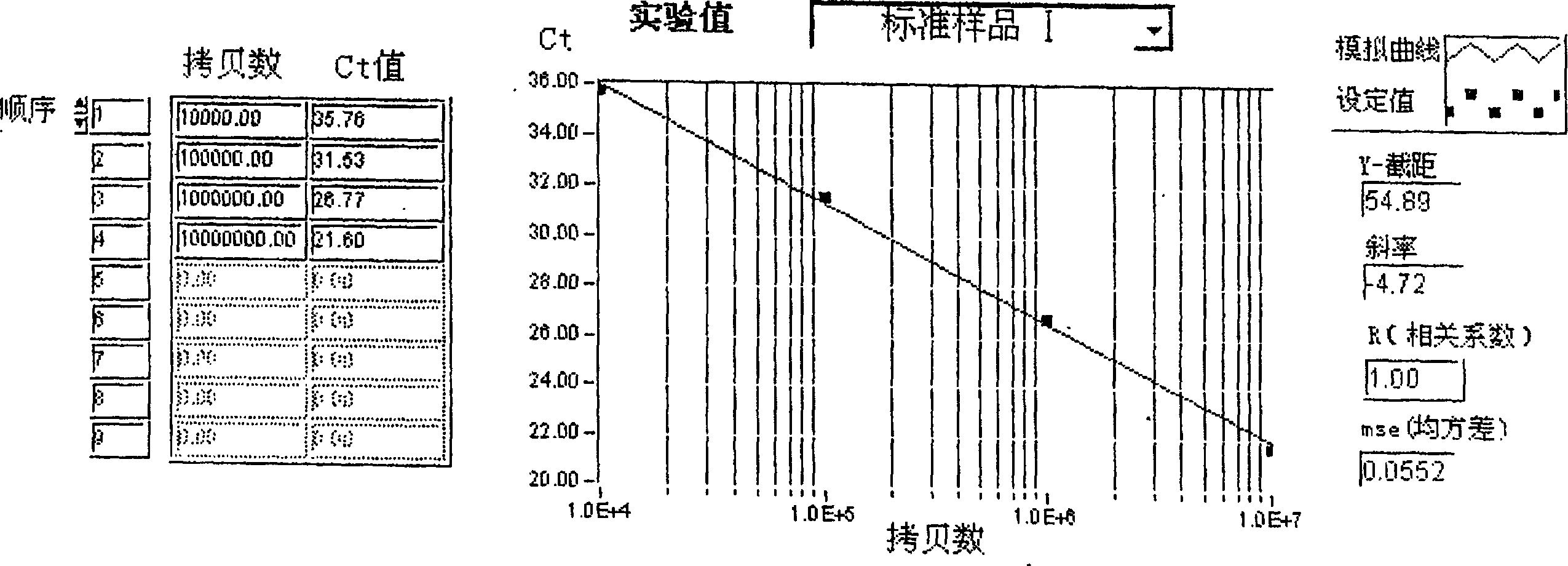
[0173] The test results are shown in Table 4, Figure 4 It can be seen that the test results of the two are basically the same:
[0174] sample
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com