Bromhexine hydrochloride oral disintegrant tablet
A technology for bromhexine hydrochloride and oral disintegrating tablets is applied in the field of bromhexine hydrochloride oral fast disintegrating tablets and their preparation, which can solve the problems of low dissolution rate and dissolution rate of ordinary tablets, slow release and absorption, and biological problems. Poor utilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
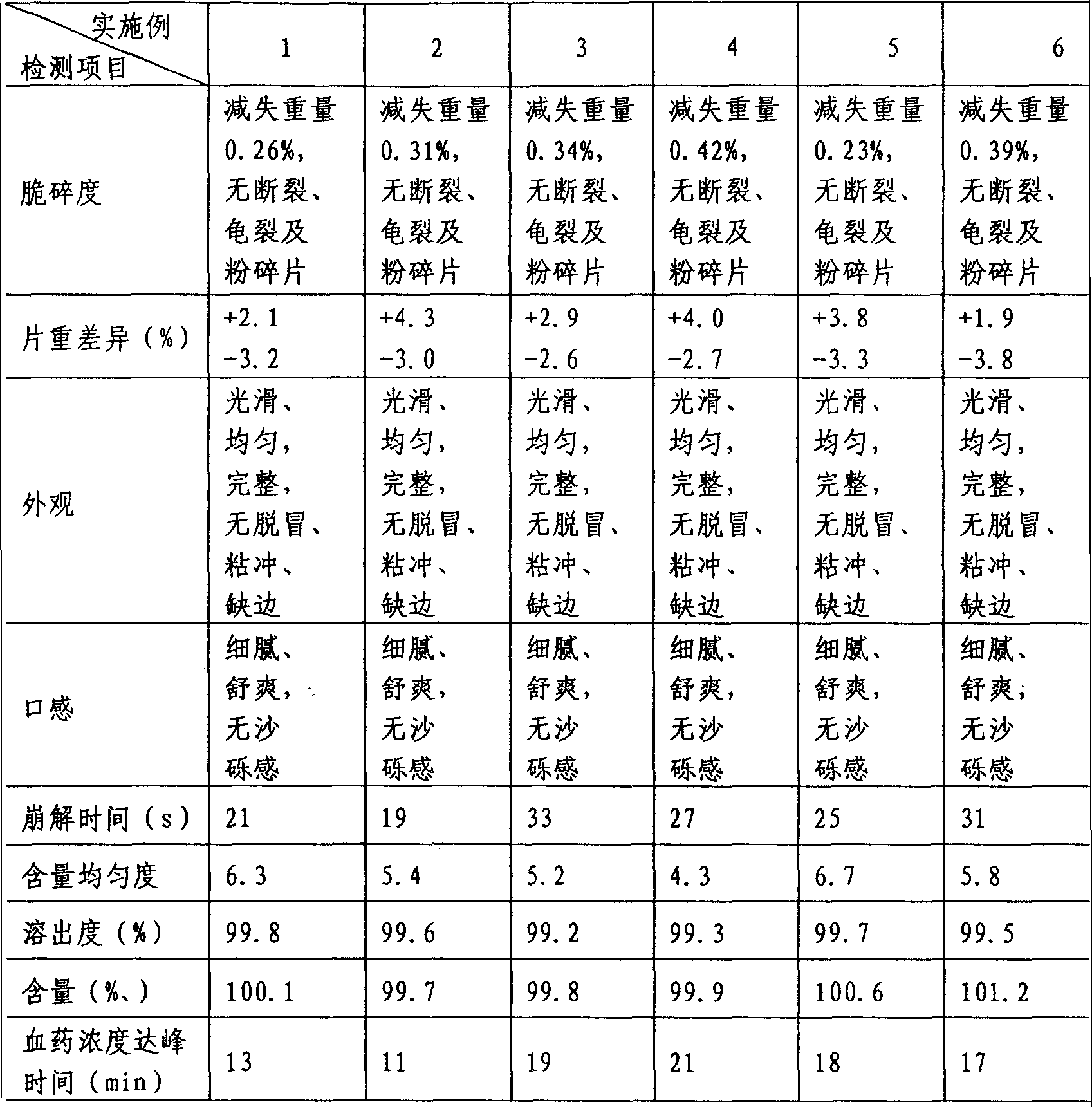
Embodiment 1
[0037] Prescription: bromhexine hydrochloride 8g
[0038] Macrogol 6000 10g
[0039] Macrogol 4000 10g
[0040] Mannitol 20g
[0041] Microcrystalline Cellulose 60g
[0042] Low-substituted hydroxypropyl cellulose 5g
[0043] Croscarmellose Sodium 10g
[0044] Cross-linked polyvinylpyrrolidone 5g
[0045] Stevia 3g
[0046] Micronized silica gel 1g
[0048] Makes 1000 pieces
[0049] Preparation method: Take the prescribed amount of polyethylene glycol 6000 and polyethylene glycol 4000, mix them evenly, heat at 90-100°C to melt, add the prescribed amount of bromhexine hydrochloride, stir well to form a molten mixture, and squeeze it with a plunger The press extrudes the molten mixture in granular form, cools it rapidly into a solid, crushes it, passes through an 80-mesh sieve, and passes through an 80-mesh sieve with the prescribed amount of microcrystalline cellulose, low...
Embodiment 2
[0051] Prescription: bromhexine hydrochloride 8g
[0052] Macrogol 6000 5g
[0053] Macrogol 4000 10g
[0054] Mannitol 50g
[0055] Microcrystalline Cellulose 25g
[0056] Low-substituted hydroxypropyl cellulose 10g
[0057] Croscarmellose Sodium 5g
[0058] Cross-linked polyvinylpyrrolidone 10g
[0059] Stevia 1g
[0060] Micronized silica gel 2g
[0061] Magnesium stearate 0.5g
[0062] Makes 1000 pieces
[0063] Preparation method: take the prescribed amount of polyethylene glycol 6000 and polyethylene glycol 4000, mix them evenly, heat at 60-80°C to melt, add the prescribed amount of bromhexine hydrochloride, stir well to form a molten mixture, and squeeze it with a plunger The press extrudes the molten mixture in granular form, cools it rapidly into a solid, crushes it, passes through an 80-mesh sieve, and passes through an 80-mesh sieve with the prescribed amount of microcrystalline cellulose, lo...
Embodiment 3
[0065] Prescription: bromhexine hydrochloride 8g
[0066] Macrogol 6000 10g
[0067] Macrogol 4000 4g
[0068] Mannitol 40g
[0069] Microcrystalline Cellulose 35g
[0070] Low-substituted hydroxypropyl cellulose 10g
[0071] Croscarmellose Sodium 10g
[0072] Cross-linked polyvinylpyrrolidone 10g
[0073] Stevia 1g
[0074] Micronized silica gel 1.5g
[0076] Makes 1000 pieces
[0077] Preparation method: Take the prescribed amount of polyethylene glycol 6000 and polyethylene glycol 4000, mix them evenly, heat at 85-95°C to melt, add the prescribed amount of bromhexine hydrochloride, stir well to form a molten mixture, and squeeze it with a plunger The press extrudes the molten mixture in granular form, cools it rapidly into a solid, crushes it, passes through an 80-mesh sieve, and passes through an 80-mesh sieve with the prescribed amount of microcrystalline cellulose, low-substituted hydroxypropyl cellulose, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com