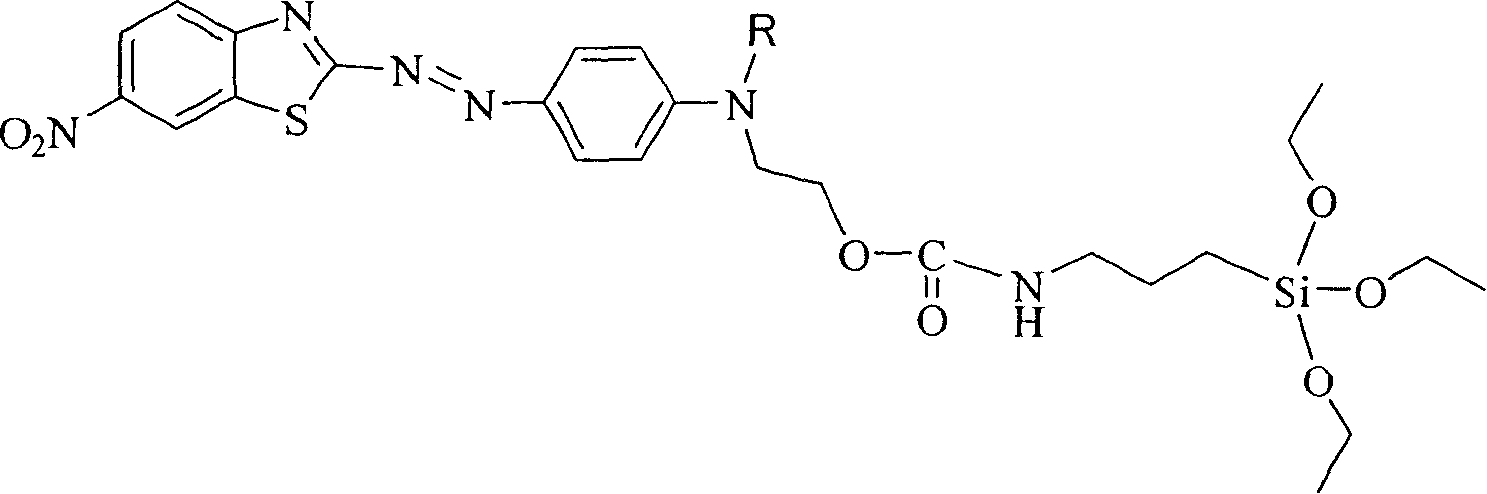
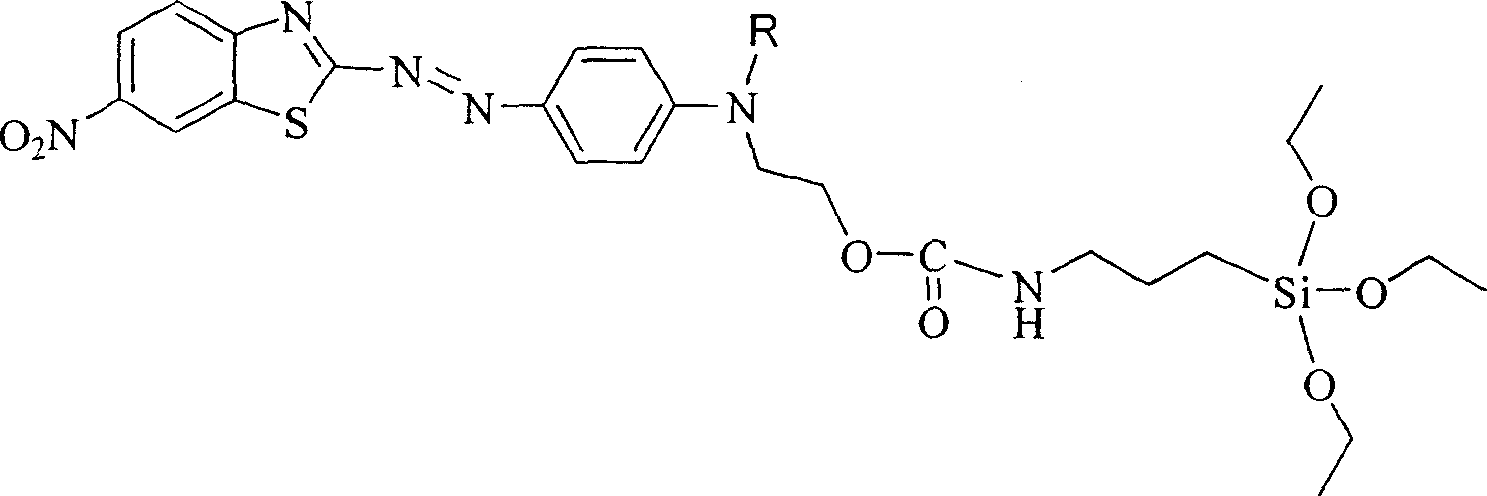
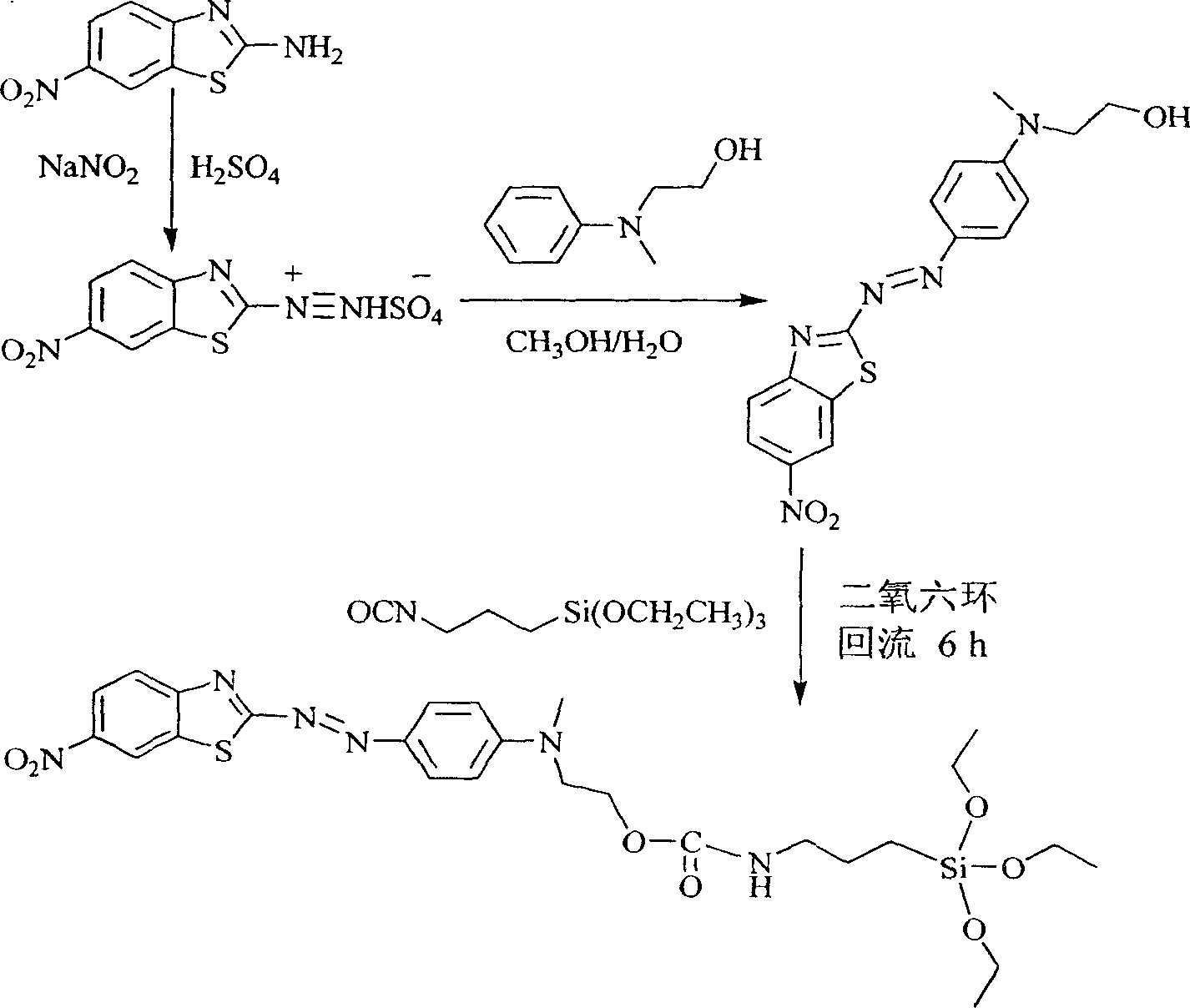
Siloxane dye comprising benzothiazole heterocycle and its synthesis method
A technology for the synthesis of benzothiazoles, which is applied in the direction of azo dyes, organic dyes, monoazo dyes, etc., can solve the problems of few and difficult preparation of azo molecules, and achieve high yield, convenient purification and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] R in Structural Formula 1 is a methyl group. The synthetic route of the siloxane dye containing benzothiazole heterocycle is as follows:
[0020]
[0021] The synthesis method is:
[0022] Slowly add 3.3g (48mmol) of sodium nitrite in batches to 40ml of 98% concentrated sulfuric acid, control the temperature below 20°C, stir to dissolve it completely, then cool to 0-5°C to obtain nitrosyl sulfuric acid. Then add 50ml of glacial acetic acid to 7.8g (40mmol) of 2-amino-6-nitrobenzothiazole, stir to form a paste and cool to below 5°C, and then quickly inward under the condition that the reaction temperature is lower than 20°C Add dropwise nitrosyl sulfuric acid, react for 10 minutes after adding, then add 6.1g (40mmol) N-methyl-N-hydroxyethylaniline and 100ml volume ratio of methanol and water mixed solution of 2 / 1, use hydrogen The sodium oxide solution adjusted the pH value to 5-6 and continued the reaction for 0.5 hour. Suction filtration, the filter cake was wash...
Embodiment 2
[0025] R in Structural Formula 1 is ethyl. The synthetic route of the siloxane dye containing benzothiazole heterocycle is as follows:
[0026]
[0027] The synthesis method is:
[0028] Slowly add 2.8g (40mmol) of sodium nitrite in batches to 40ml of 98% concentrated sulfuric acid, control the temperature below 20°C, stir to dissolve it completely, then cool to 0-5°C to obtain nitrosylsulfuric acid. Add 24ml of glacial acetic acid, 16ml of concentrated phosphoric acid, 8ml of propionic acid and 8ml of formic acid to 7.8g (40mmol) of 2-amino-6-nitrobenzothiazole, stir to form a paste and cool to below 5°C, then ensure the reaction Under the condition that the temperature is lower than 20°C, nitrosyl sulfuric acid is quickly added dropwise inwardly, and reacted for 1 hour after the addition is completed, then add 7.4g (45mmol) N-ethyl-N-hydroxyethylaniline and 120ml volume ratio of 2 / 1 mixed solution of methanol and water, adjust the pH value to 5-6 with ammonia water and...
Embodiment 3
[0040] R in the general structural formula 1 is a benzene ring. The synthetic route of the siloxane dye containing benzothiazole heterocycle is as follows:
[0041]
[0042] The synthesis method is:
[0043] Slowly add 2.8g (40mmol) of sodium nitrite in batches to 40ml of 98% concentrated sulfuric acid, control the temperature below 20°C, stir to dissolve it completely, then cool to 0-5°C to obtain nitrosylsulfuric acid. Then add 30ml of concentrated sulfuric acid to 7.8g of 2-amino-6-nitrobenzothiazole, stir to form a paste and cool to below 5°C, then quickly dropwise add disulfide under the condition that the reaction temperature is lower than 20°C. Nitrosulfuric acid, after adding, reacted for 5 hours, then added 16.0g (80mmol) N-ethyl-N-hydroxyethylaniline and 200ml volume ratio of methanol and water mixed solution of 2 / 1, adjusted with sodium carbonate solution The reaction was continued for 5 hours when the pH value reached 5-6. Suction filtration, the filter cake ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com