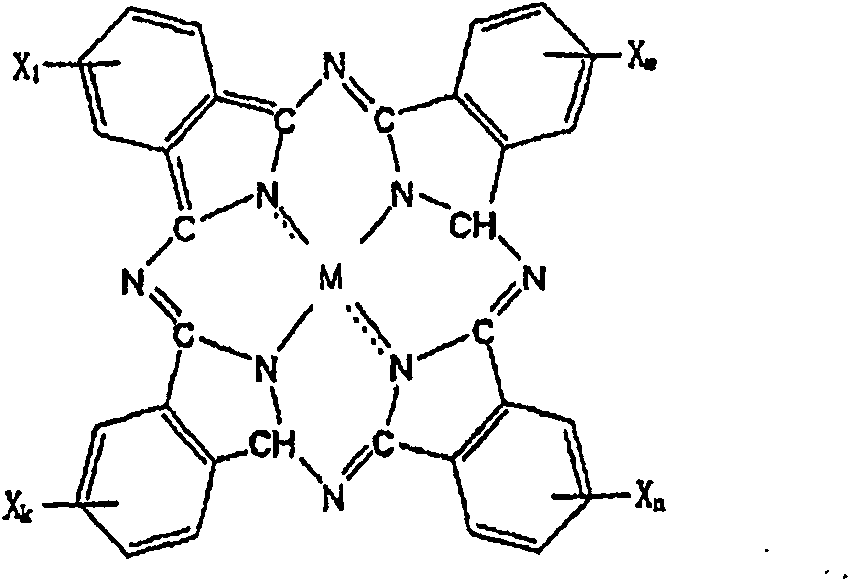
Process and apparatus for preparing metal or nonmetal phthalocyanine
A non-metal and metal technology, applied in the field of preparing metal or non-metal phthalocyanine, can solve problems such as difficult mass production of phthalocyanine, unsuitable for mass production and commercialization of phthalocyanine, unfavorable processing efficiency and environmental management
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of Copper Phthalocyanine
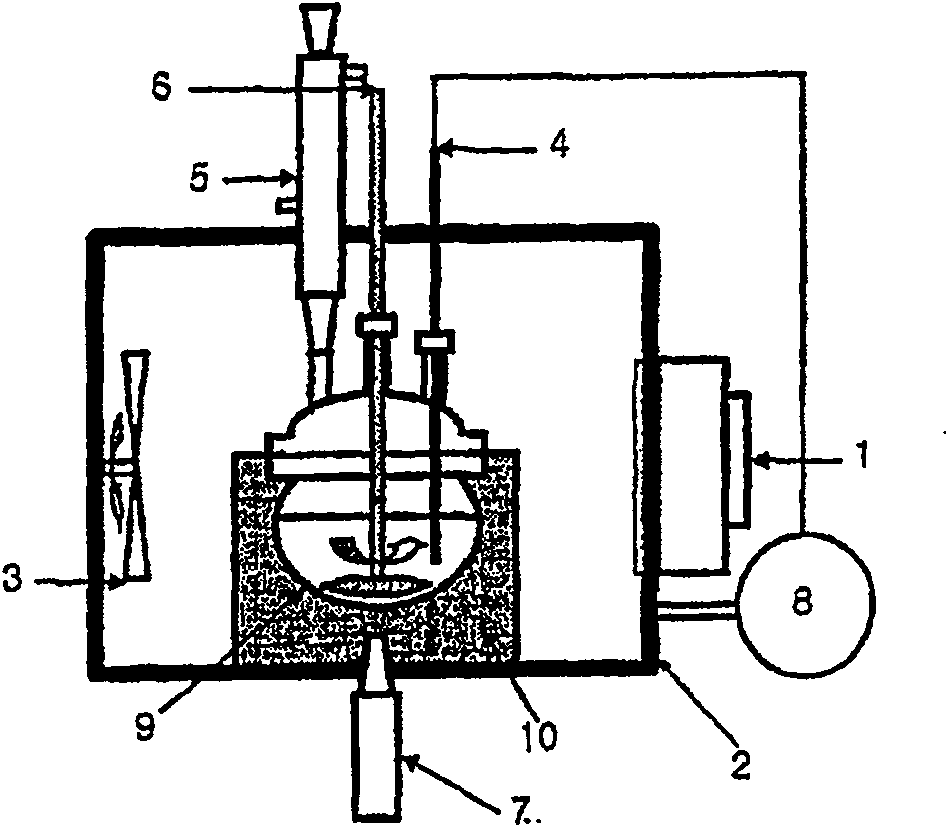
[0041] This example was carried out in a solvent-based device according to the invention. Specifically, 42 g of anhydrous phthalic acid, 49 g of urea, 7 g of copper chloride, 0.1 g of ammonium molybdate, and 100 g of alkylbenzene were introduced into a pyrex glass container 9, followed by microwaves at 28 kHz and ultrasonic energy at 250 W The reactant was uniformly stirred for 3 hours at 180-185° C. to prepare copper phthalocyanine. During the reaction, the temperature of the reactants was precisely controlled within a deviation range of ±1° C. using the PID temperature controller 8, and thus a microwave power of 100-3,000 W was maintained. Microwave and ultrasonic energy are used simultaneously from the initial stages of the reaction. After completing the preparation, the solvent was removed by distillation under reduced pressure. Add dry copper phthalocyanine to 500ml of 5% sulfuric acid solution, treat with acid at 85°C for ...
Embodiment 2
[0043] Preparation of other phthalocyanines
[0044] Various phthalocyanines were prepared in the same manner as shown in Example 1, but using 1,2-dicyanobenzene instead of anhydrous phthalic acid, and using a compound selected from titanium, iron, cobalt, aluminum, manganese, tin and Metal salts of nickel were used in place of equivalent weight of copper chloride as the metal source (in the case of non-metallic phthalocyanines, no metal source was used).
Embodiment 3
[0046] Preparation of Copper Phthalocyanine
[0047] 300 ml of alumina microbeads with a diameter of 30 mm were charged into an attritor 12 equipped with a microwave generating device, and then 42 g of anhydrous phthalic acid, 49 g of urea, 7 g of cuprous chloride and 0.1 g of ammonium molybdate were added thereto. The reactant was heated to 120°C at a rate of 10°C / min, while being stirred at 300-400rpm by the stirrer 17, and further heated to the final preparation temperature (180°C) at a rate of 5°C / min. The final preparation temperature was maintained for 3 hours while uniformly stirring the mixture to prepare copper phthalocyanine. During the reaction process, the temperature of the reactants is precisely controlled within a deviation range of ±1°C using a PID temperature controller, and a microwave power of 100-4,000W is maintained. After finishing the preparation, the attritor was cooled to 60° C., and 500 ml of 5% sulfuric acid solution was added thereto. After stirri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com