Pharmaceutical composition containing valibose, its preparation method and application
A velibose and composition technology, which is applied in the field of velibose-containing compositions, can solve the problems of no velibose preparation method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] 1. Preparation of raw material Validamine
[0089] The raw material Validamine can be prepared by methods disclosed in prior art documents, for example: methods described in J. Antibiotics 24: 57-58, 1971 and Chem. Lctt., 1989, 725-728 (Harii, s.j.T.Iwasa & Y.Kameda), In the preparation process of the present invention, the preparation of the raw material Validamine is to obtain validoxylamineA by hydrolysis of Jinggangmycin A (purchased from Zhejiang Qianjiang Biochemical Co., Ltd., content 60%), and then obtain validoxylamineA from validoxylamineA and N-bromosuccinyl Amine (NBS) reaction to obtain validamine. The reaction formula is as follows:
[0090]
[0091] The reaction product was treated with CG-50NH 4 + Weak acid type cationic resin column, 0.1N ammonia water elution separation to obtain pure Validamine and Valienamine.
[0092] 2. Preparation of velibose in crystal form
[0093] The reaction formula is as follows:
[0094]
[0095] In 2600 ml of a...
Embodiment 1
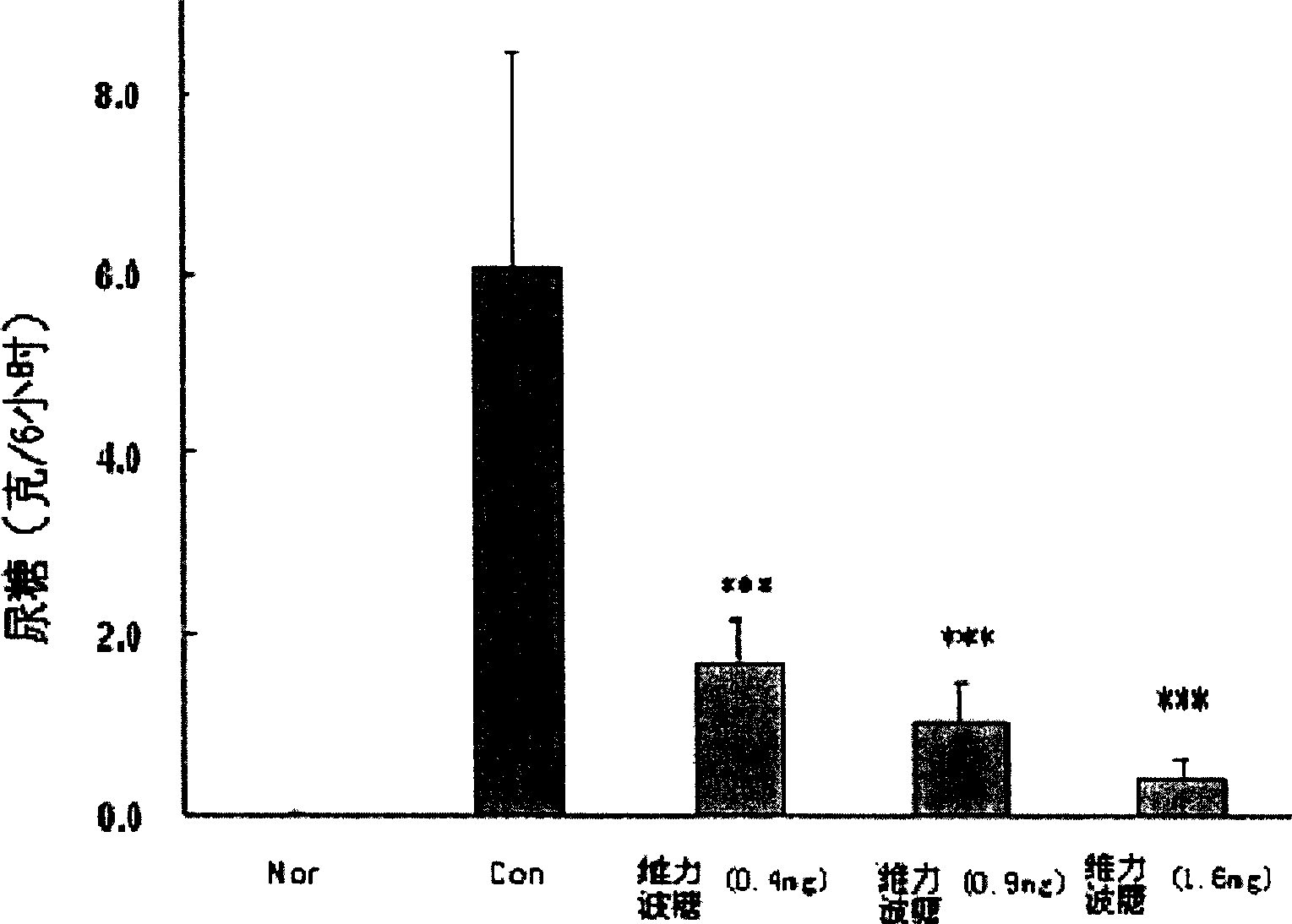
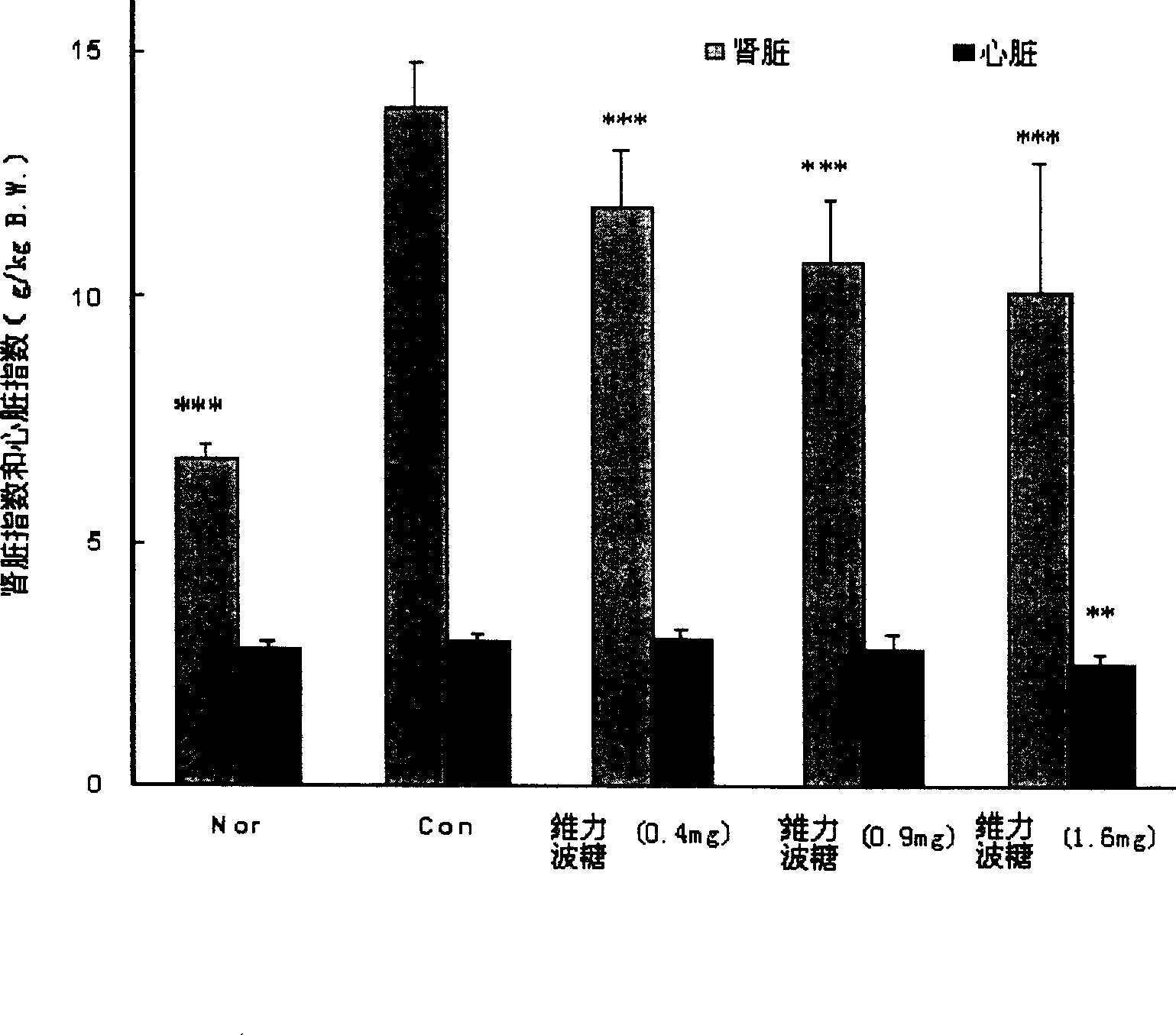
[0112] Taking Velibose as the test drug, the general condition, blood sugar, fructosamine, urine sugar, blood NAG enzyme activity, serum urea nitrogen and creatinine content, Renal pathological changes and the effects of lipids (including triglycerides, cholesterol and free fatty acids) and cardiac index.
[0113] In the long-term experiment (5 weeks) of streptozotocin (STZ) diabetic rats fed with high-sugar diet, when the daily dose of rats was ≥0.4 mg / kg body weight, (1) Velibose made three Multiple symptoms were significantly relieved and food intake was reduced, indicating that velibose can be used to treat overeating and the obesity caused by it; (2) velibose significantly reduced fasting blood sugar, non-fasting blood sugar, serum Fructosamine and urine sugar indicate that velibose can improve the glucose metabolism of hyperglycemic animals and can be used to prevent or treat diabetes; (3) velibose can significantly improve serum NAG enzyme activity, serum urea nitrogen ...
Embodiment 2
[0182] I prescription
[0183]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com