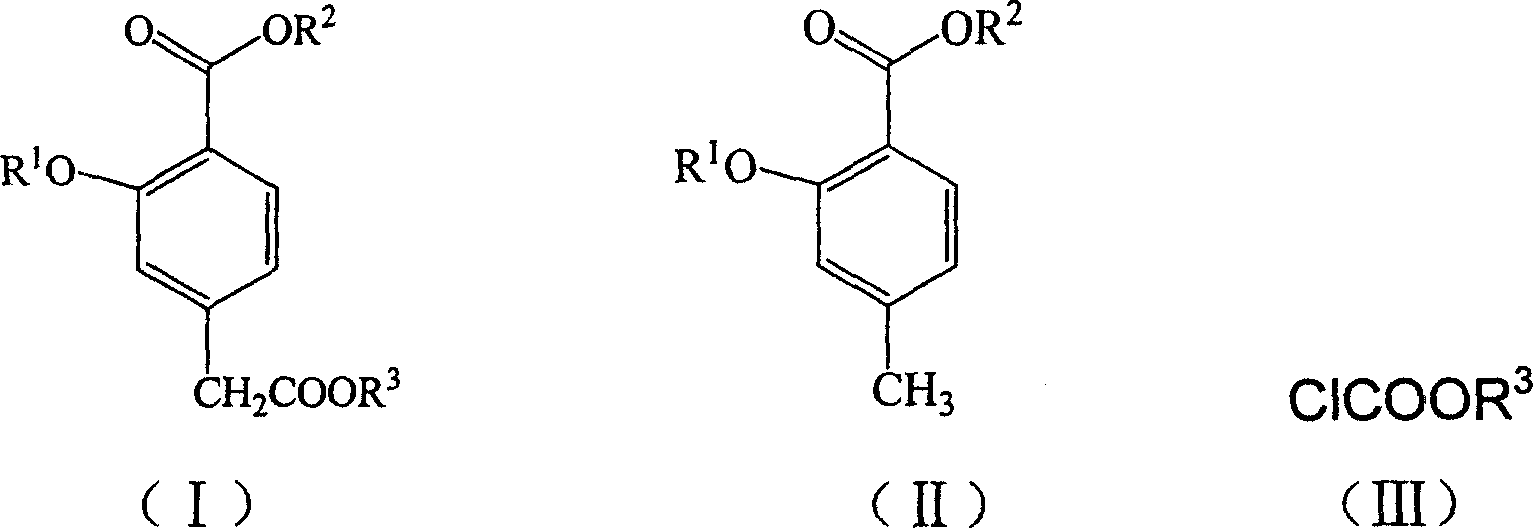
3- alkoxy -4-carbalkoxyphenylacetate and 3-alkoxy-4-carbalkoxyphenylacetic acid synthesis method
A technology for alkoxycarbonyl phenylacetate and a synthesis method, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of carboxylate/lactone, etc., can solve the problems of long production cycle, many steps, and high total cost , to achieve the effect of short synthesis route, high reaction yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of embodiment 1 3-ethoxy-4-methylbenzoic acid ethyl ester
[0024] Put 30.4g (0.2mol) of 4-methylsalicylic acid, 92.5g (0.6mol) of diethyl sulfate and 2g of tetrabutylammonium bromide in a mixed solution of 500mL of dichloromethane and 500mL of water, at room temperature (25°C ) was stirred for 20h, and the dichloromethane layer was separated after the reaction was completed, and the aqueous layer was extracted with dichloromethane (150mL×2), the organic phases were combined, washed with water, and concentrated to obtain the product 3-ethoxy-4-methylbenzoic acid ethyl 36 g of ester, yield 86.5%. The next reaction was carried out without purification.
Embodiment 2
[0025] Example 2 Synthesis of 3-ethoxyl-4-ethoxycarbonylphenylacetic acid ethyl ester
[0026] Under the protection of nitrogen, add 9.7g (0.095mol) of diisopropylamine into 80mL of tetrahydrofuran, stir, and after the system drops to -80°C, add 60mL (0.096mol) of n-hexane solution of butyllithium, stir for 30min, add dropwise 1 The tetrahydrofuran solution of 10 g (0.048 mol) of the obtained 3-ethoxy-4-methylbenzoic acid crude product was stirred for 30 min, then the tetrahydrofuran solution of 7.8 g (0.071 mol) of ethyl chloroformate was added, and after stirring for 3 h, added 5% ammonium chloride solution, raised to room temperature, adjusted to pH = 7 with 2N HCl, separated the organic layer, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, concentrated to obtain the product 3-ethoxy-4-ethane 22 g of ethyl oxycarbonyl phenylacetate, the product was directly carried out to the next step without purification.
Embodiment 3
[0027] Example 3 Synthesis of 3-ethoxyl-4-ethoxycarbonylphenylacetic acid
[0028] Dissolve 16 g of the crude ethyl 3-ethoxy-4-ethoxycarbonylphenylacetate obtained in Example 2 in 80 mL of 95% ethanol, lower the reaction solution to 5-10 °C, and then add 2N sodium hydroxide solution in batches to pH Until the value no longer changes, control the reaction temperature at 5-15°C. After the reaction was completed, the reaction solution was adjusted to neutral with 6N hydrochloric acid, concentrated ethanol, then added 50ml of water, extracted with dichloromethane (25mL×2), separated the water layer, and adjusted the pH to 3.5-4 with 6N HCl under ice bath. CH 2 Cl 2 Extraction (30mL×2), the organic layer was successively washed with water (30mL×2), saturated brine (30mL×2), dried over anhydrous sodium sulfate, and concentrated to obtain a yellow solid, which was decolorized and recrystallized with toluene to obtain 6.0 g of a white solid . The overall yield is 58%. mp: 75-76°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com