Renaturation of reconstituted human bone protein-1 and making method of its preparation
An osteogenic protein and preparation technology, applied in the field of biotechnology and pharmacy, can solve the problems of high cost, limited use, low yield and the like, and achieve the effect of simple and easy renaturation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
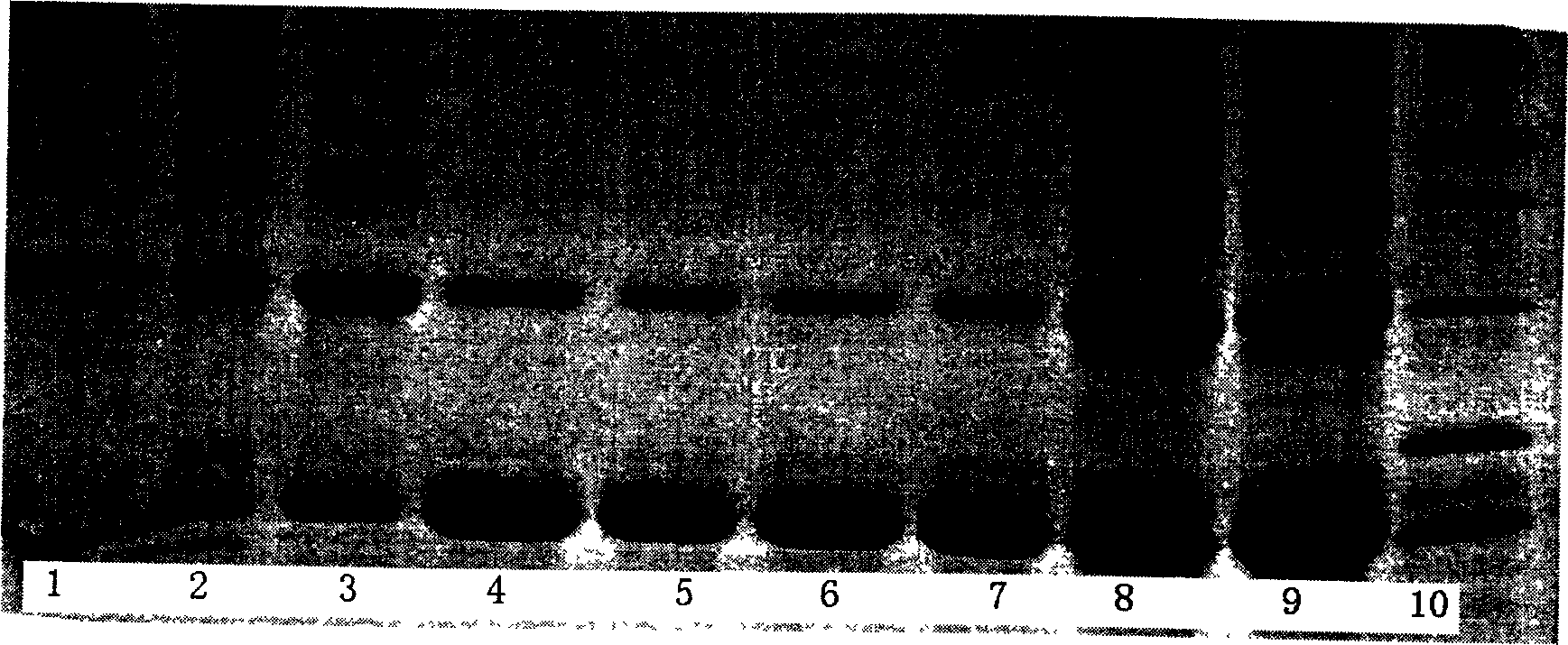
[0034] The rhOP-1 engineered bacteria are fermented, collected by centrifugation, lysed, and washed to obtain inclusion bodies. The inclusion bodies are denatured and dissolved by 8 mol / L urea, and then ion exchange (or / and hydrophobic, affinity and molecular exclusion) layer After analysis, collection and purification to a purity of over 95%, it was diluted to 200-600 μg / ml. Then put it into a conventionally treated dialysis bag (molecular weight cut-off of 10,000), in 6 mol / L of urea, 0.05 g / L of polyethylene glycol 4000, 0.4-0.6 mol / L of arginine, 20 mmol In the renaturation solution of phosphate buffer (pH8.0~9.5) in the renaturation solution of phosphate buffer (pH8.0~9.5), air oxidative renaturation at room temperature for 24~96 hours, and then undergo further ion exchange chromatography and size exclusion purification to make the dimer purity of the target protein reach 90 %above. Then 5 mol / L urea, 4 mol / L urea, 2 mol / L urea, and 1 mol / L urea were dialyzed into 20 mmo...
Embodiment 2
[0038] The rhOP-1 engineered bacteria were fermented, collected by centrifugation, lysed, and washed to obtain inclusion bodies, which were denatured and dissolved by 6 mol / L guanidine hydrochloride; ion exchange (or / and hydrophobic, affinity and molecular exclusion) After chromatographic collection and purification reach more than 95% of the purity, in the guanidine hydrochloride of 4 mol / liter, and the arginine of 0.2~0.5 mol / liter, the copper ion of 0.1 micromol / liter~0.1 mmol / liter (chloride copper or copper sulfate); 20 mmol / L phosphate refolding solution (pH8.0~9.0) was refolded at room temperature for 24 to 96 hours, and then further purified by ion exchange chromatography (or / and reversed phase, hydrophobic, Non-specific affinity and molecular exclusion) make the purity of the target protein reach more than 90%. The obtained sample is directly compounded with the carrier material (collagen, absorbent gelatin sponge, chitosan or coral powder, one of the synthetic polyme...
Embodiment 3
[0040] The inclusion bodies are denatured and dissolved with 8 mol / L urea, collected and purified by ion exchange chromatography to reach a purity of over 95%, and then diluted to 100-600 μg / ml. Then put it into a conventionally treated dialysis bag (molecular weight cut off is 10,000), and directly oxidize it in air at room temperature in the refolding solution of 6 mol / L urea and 20 mmol / L phosphate buffer (pH9.0~9.5). After refolding for 24-96 hours, further ion-exchange chromatography and molecular exclusion purification are performed to make the dimer purity of the target protein reach more than 90%. Then 5 mol / L urea, 4 mol / L urea, 2 mol / L urea, and 1 mol / L urea were dialyzed into 20 mmol / L phosphate buffer solution, and freeze-dried to obtain rhOP-1 freeze-dried powder. Store at -20°C after ethylene oxide sterilization. Alternatively, the target protein with a dimer purity of more than 90% is directly compounded with a certain amount of absorbent gelatin sponge materia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com