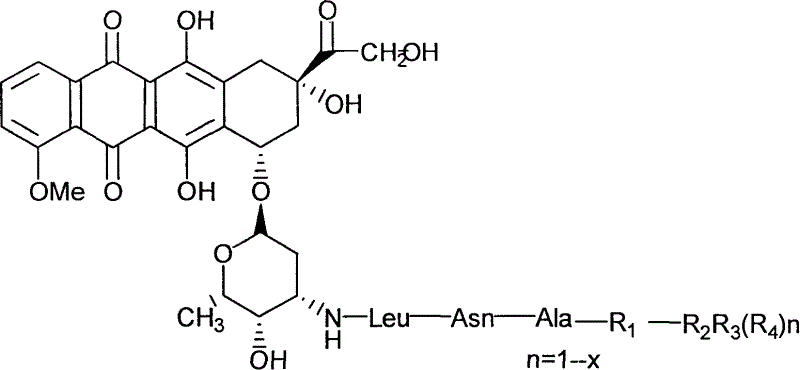
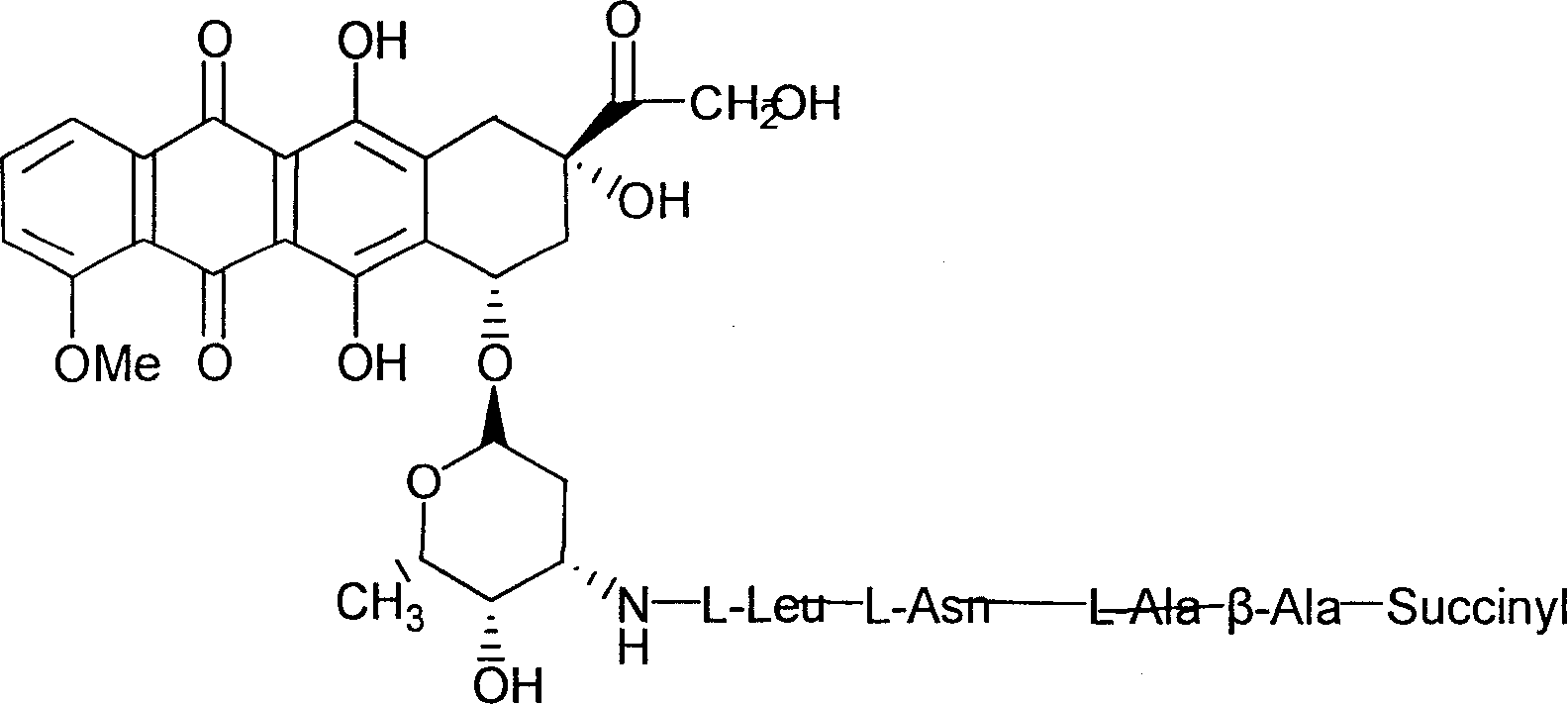
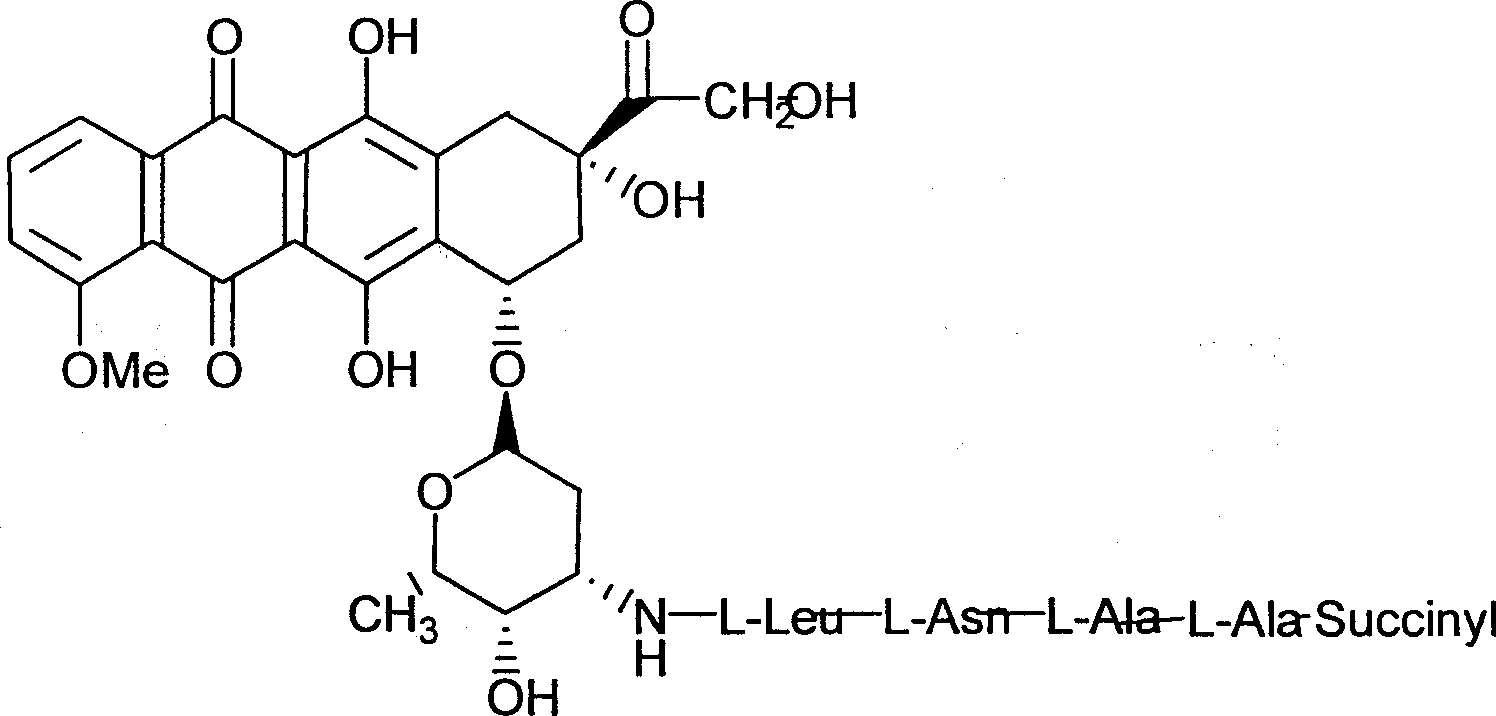
Adriamycin derivative and its preparing method and use
A technology of doxorubicin and its derivatives, which is applied in the field of doxorubicin derivatives, can solve the problems of no practical application value, unstable amino protecting group, and inability to make injections, so as to improve bioavailability and clearly target The effect of tropism and not easy to hydrolyze
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of SUC-L-ALA-L-ALA-L-ASN-LEU-DOX (SD-1)
[0029] (Succinyl L-alanyl L-alanyl L-asparaginyl L-leucyl doxorubicin) (SD-1)
[0030] 1. Synthesis of BOC-L-ALA-L-ALA-L-ASN-L-LEU-OH
[0031] (tert-butoxycarbonyl)-L-alanyl-L-alanyl-L-asparaginyl-L-leucine)
[0032] a. Preparation of Boc-L-ASN-LEU-OBZL
[0033] Boc-L-ASN (tert-butoxycarbonyl-L-asparagine) 23.2g (100mmol), L-LEU-OBZL (L-leucine benzyl ester) 24.3g (110mmol), HOBT (1-hydroxybenzo Triazole) 14.8g (110mmol), THF (tetrahydrofuran) 300ml, dissolve together, cool down in ice water, add DCC (N,N`-dicyclohexylcarbodiimide) 22.7g (110mmol), react for 4hr, filter to remove DCU (N,N'-dicyclohexyl urea), THF was distilled off under reduced pressure, 200ml of ethyl acetate was added, impurities were washed with acid, dried and concentrated, 39g of the product recrystallized in ethyl acetate, yield 90%. The structure of the product was confirmed by mass spectrometry. + 436.1 was directly used in the n...
Embodiment 2
[0053] Embodiment 2, the synthesis of SUC-β-ALA-L-ALA-L-ASN-LEU-DOX (SD-2)
[0054] (Succinyl β-alanyl L-alanyl L-asparaginyl L-leucyl doxorubicin) (SD-2)
[0055] 1. Synthesis of BOC-β-ALA-L-ALA-L-ASN-L-LEU-OH
[0056] a. Preparation of Boc-L-ASN-LEU-OBZL
[0057] Boc-L-ASN23.2g (100mmol), L-LEU-OBZL24.3g (110mmol), HOBT14.8g (110mmol), THF300ml, dissolve together, cool down in ice water, add DCC22.7g (110mmol), react for 4hrs, filter DCU was removed, THF was distilled off under reduced pressure, 200 ml of ethyl acetate was added, impurities were washed with acid, dried and concentrated, 39 g of the product recrystallized in ethyl acetate, yield 90%. The structure of the product was confirmed by mass spectrometry. + 436.1 was directly used in the next reaction without further purification.
[0058] b. Preparation of Boc-L-ALA-L-ASN-LEU-OBZL
[0059] Weigh the product Boc-L-ASN-LEU-OBZL 34.8g (80mmol) from the previous step, dissolve it in 200ml of 1NHCL ethyl acetate, re...
Embodiment 3
[0077] Example 3 Synthesis of other derivatives of β-ALA-L-ALA-L-ASN-LEU-DOX
[0078] Based on β-ALA-L-ALA-L-ASN-LEU-DOX, it can easily pass through the reaction of acid chlorides and amino groups of various acids, the condensation reaction of amino groups and various acids, and the reaction of acid anhydrides and amino groups, The reaction of active esters and amino groups, etc., to synthesize various derivatives of β-ALA-L-ALA-L-ASN-LEU-DOX, including various natural and non-natural derivatives, various carboxylic acids, sulfonic acids, phosphoric acids Class derivatives, these reactions are easy to realize for synthetic workers, and are simply stated in the present invention.
[0079] Weigh β-ALA-L-ALA-L-ASN-LEU-DOX 0.912g (1mmol), BocGlu (obut) (tert-butyloxycarbonylglutamic acid tert-butyl ester) 0.33g (1.1mmol), if in the reaction bottle, After adding 5ml of DMF, cooling in ice water, assuming HBTU0.42g (1.1mmol), reacted for 2 hours, poured into water to precipitate, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com