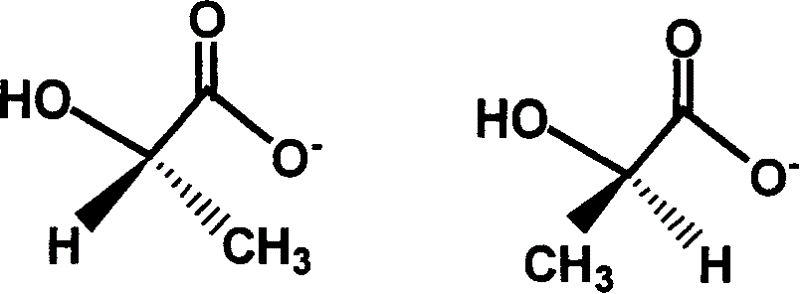
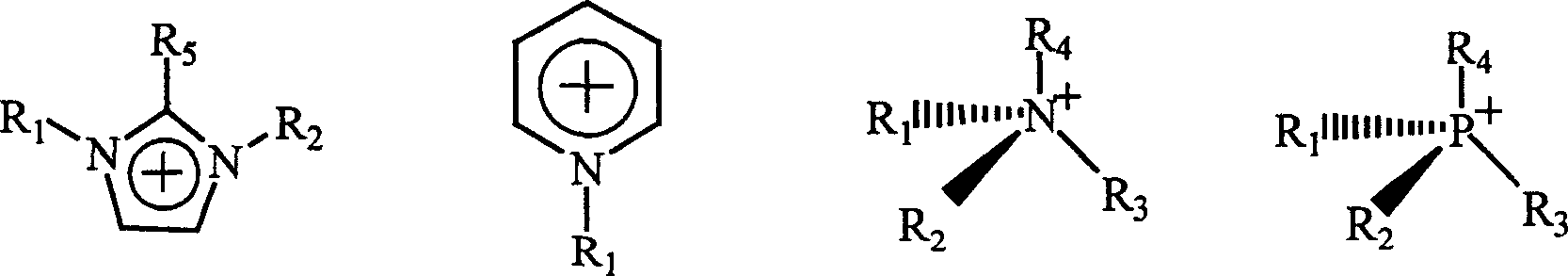
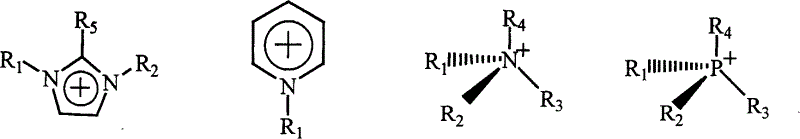Chiral ionic liquid and its preparing method
A chiral ionic liquid and ionic liquid technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. In the initial stage, the types of chiral ionic liquids are limited, etc., to achieve large structural diversity and designability, which is conducive to large-scale industrial production and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of 1-ethyl-3-methylimidazole L-lactate
[0029] in N 2 Under protection, N-methylimidazole (41g, 0.50mol) and bromoethane (54.5g, 0.55mol) were added in a three-neck round bottom flask equipped with a magnetic rotor and a reflux condenser, and the solvent benzene (100g) was added, Heated to 80°C in an oil bath and stirred for 48 hours. After cooling, the ionic liquid and the solvent were separated. After separation, the lower liquid was washed three times with ethyl acetate to remove residual reactants, and then washed with ether to remove residual ethyl acetate, followed by vacuum distillation at 70°C for 48 hours After that, 1-ethyl-3-methylimidazolium chloride was obtained. Dissolve 1-ethyl-3-methylimidazolium bromide (emimBr, 95.5g, 0.50mol) in 60g of dichloromethane, then add L-sodium lactate (56.0g, 0.50mol), in an ice bath (0°C) The reaction was stirred for 10 hours, then filtered. 90°C, 10 3 Dichloromethane was removed by evaporation on a rotary e...
Embodiment 2
[0031] Synthesis of 1-butyl-3-methylimidazole L-lactate
[0032] in N 2 Under protection, N-methylimidazole (41g, 0.50mol) and chlorobutane (50.9g, 0.55mol) were added in a three-necked round bottom flask equipped with a magnetic rotor and a reflux condenser, and the solvent toluene (80g ), heated to 70°C in an oil bath, and stirred for 72 hours. After cooling, the ionic liquid and the solvent were separated. After separation, the lower liquid was washed three times with ethyl acetate to remove residual reactants, and then washed with ether to remove residual ethyl acetate, followed by vacuum distillation at 70°C for 48 hours After that, 1-butyl-3-methylimidazolium chloride was obtained. Dissolve 1-butyl-3-methylimidazolium chloride (43.64g, 0.25mol) in 160g of acetone, then add L-potassium lactate (64.0g, 0.50mol), stir and react at 30°C for 5 hours, then filter . 80°C, 5×10 3 Evaporate in a rotary evaporator under Pa for 10 hours to remove acetone, then put it in a vacu...
Embodiment 3
[0034] Synthesis of 1,3-dihexylimidazole D-lactate
[0035] N 2 Under protection, add N-trimethylsilyl imidazole (72g, 0.50mol) and bromo-n-hexane (181.6g, 1.1mol) in the round bottom flask equipped with magnetic rotor and reflux condenser, add solvent toluene (150g) , reflux reaction at 100°C for 12 hours, after cooling, the layers were separated, and after liquid separation, the lower liquid was dissolved in dry acetonitrile, and the impurities were repeatedly extracted with n-hexane three times, and the acetonitrile was removed by vacuum distillation to obtain 1,3-dihexylimidazolium bromide Compound (hhimBr), dried at 70°C for 48 hours to obtain 1,3-dihexylimidazolium bromide. 1,3-Dihexylimidazolium bromide (158.5 g, 0.5 mol) was dissolved in 30.8 g of chloroform, then L-magnesium lactate (40.4 g, 0.20 mol) was added, stirred at room temperature for 10 hours, and then filtered. Evaporate in a rotary evaporator at 40°C and 1000Pa for 24 hours to remove chloroform, then put...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com