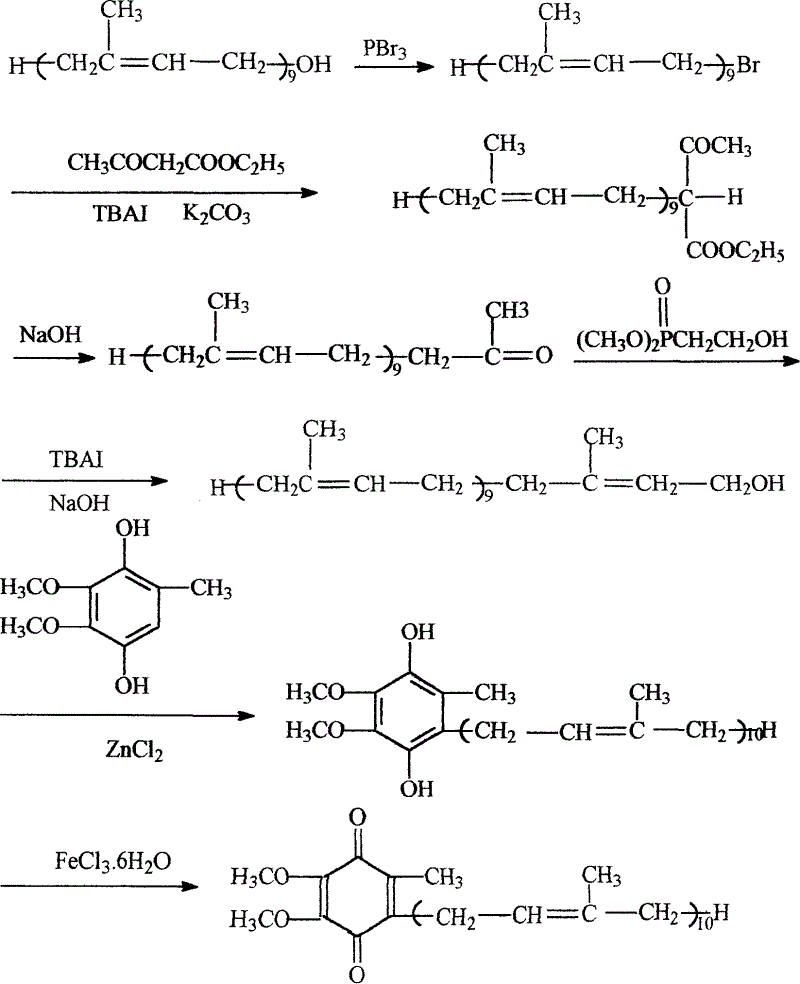
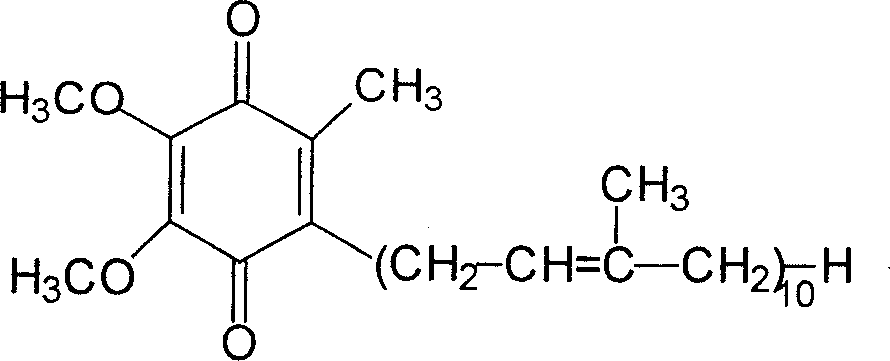
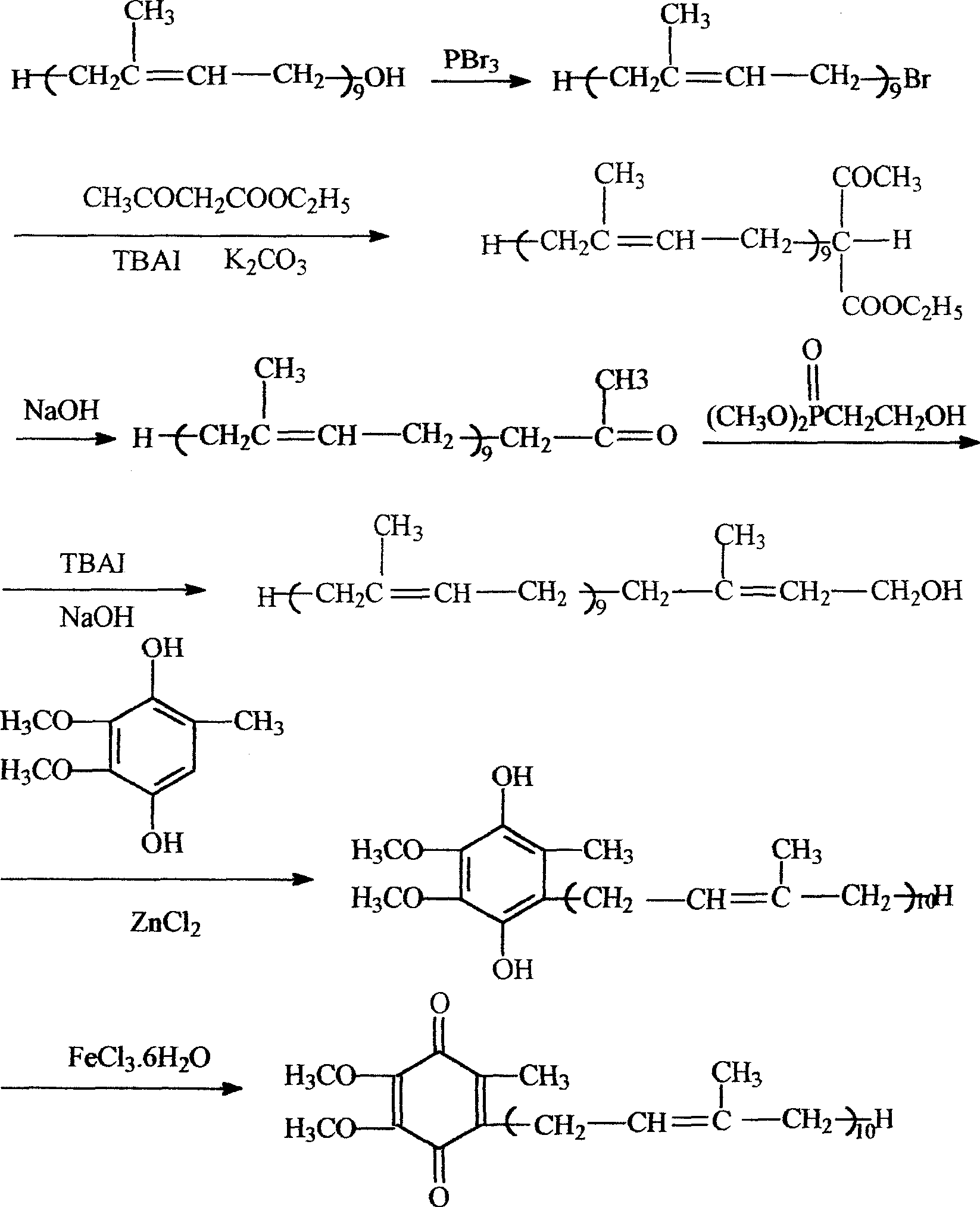
A novel process for synthesis of coenzyme Q10
A technology of coenzyme and solanesyl acetone, which is applied in the field of preparation of organic polymer compounds, can solve problems such as the reduction of the possibility of industrialization, and achieve the effects of easy control of process conditions, mild reaction conditions, and overcoming reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] Synthetic Step 1: Synthesis of Solanesyl Bromide
[0023] Chemical Structure:
[0024] Chemical name: 1-Bromo-3, 7, 11, 15, 19, 23, 27, 31, 35-nonamethyl-hexadecane-2, 6, 10, 14, 18, 22, 26, 30, 34-nonacene
[0025] Chemical formula: C 45 h 73 Br
[0026] Molecular weight: 694
[0027] Melting point: 41.5-43°C
[0028] CA registration number: (all-E)52610-77-2
[0029] Physical properties: The pure product should be a white waxy solid; the unpurified product should be a brown oil, which solidifies into a solid after refrigeration. Insoluble in water, soluble in organic solvents.
[0030] Reaction equation:
[0031]
[0032] Operation process:
[0033] Add 7.0g (10mmol) solanesol (90%) and 20ml petroleum ether (50-60°C fraction, the same below) to a three-neck flask equipped with a stirrer, a thermometer, and a dropping funnel, stir to dissolve, and then add 0.5 ml (6mmol) pyridine, under stirring at a temperature of 0-10°C, add dropwise 0.5ml (5mmol) of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com