Preventive and/or therapeutic drugs for inflammatory intestinal diseases
An inflammatory bowel disease and drug technology, applied in the field of myeloperoxidase activity inhibitors, can solve the problem of no research on whether edaravone is effective for inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0116] Below, illustrate the present invention more specifically by embodiment, the present invention is not limited to following
[0117] Example.
Synthetic example
[0118] Synthesis example: Synthesis of 3-methyl-1-phenyl-2-pyrazolin-5-one (hereinafter referred to as edaravone)
[0119] 13.0 g of ethyl acetoacetate and 10.8 g of phenylhydrazine were added to 50 ml of ethanol, and the mixture was stirred under reflux for 3 hours. After the reaction solution was left to cool, the precipitated crystals were filtered and recrystallized from ethanol to obtain 1.3 g of the title compound as colorless crystals.
[0120] Yield 67%
[0121] Melting point 127.5~128.5℃
Embodiment 1
[0123] (1) Experimental method
[0124] 1 material
[0125] The edaravone synthesized as above was dissolved in a small amount of 1N sodium hydroxide solution, and the pH was adjusted to be near neutral.
[0126] 2 Methods of glycosyl esters (DSS) inducing colitis
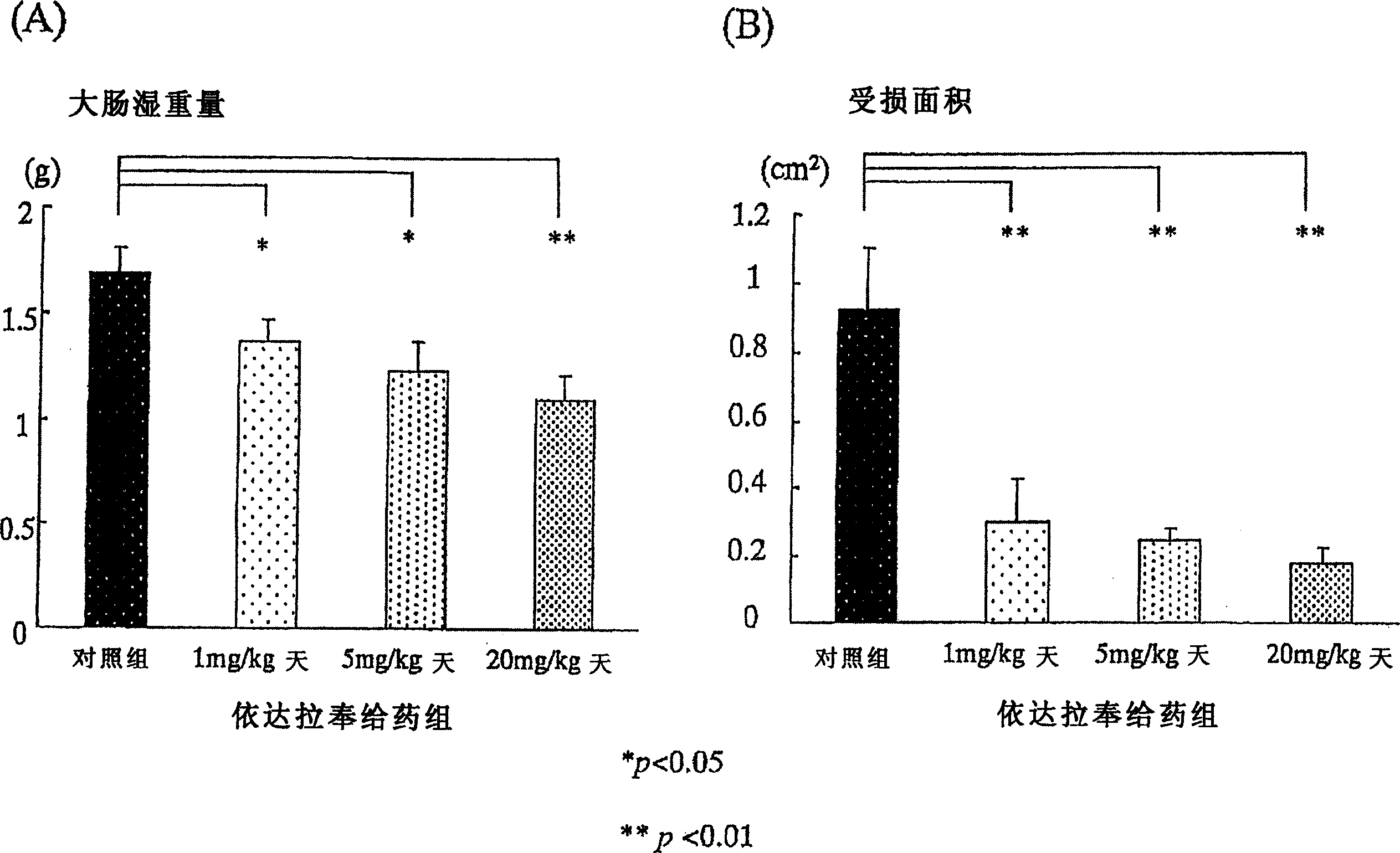
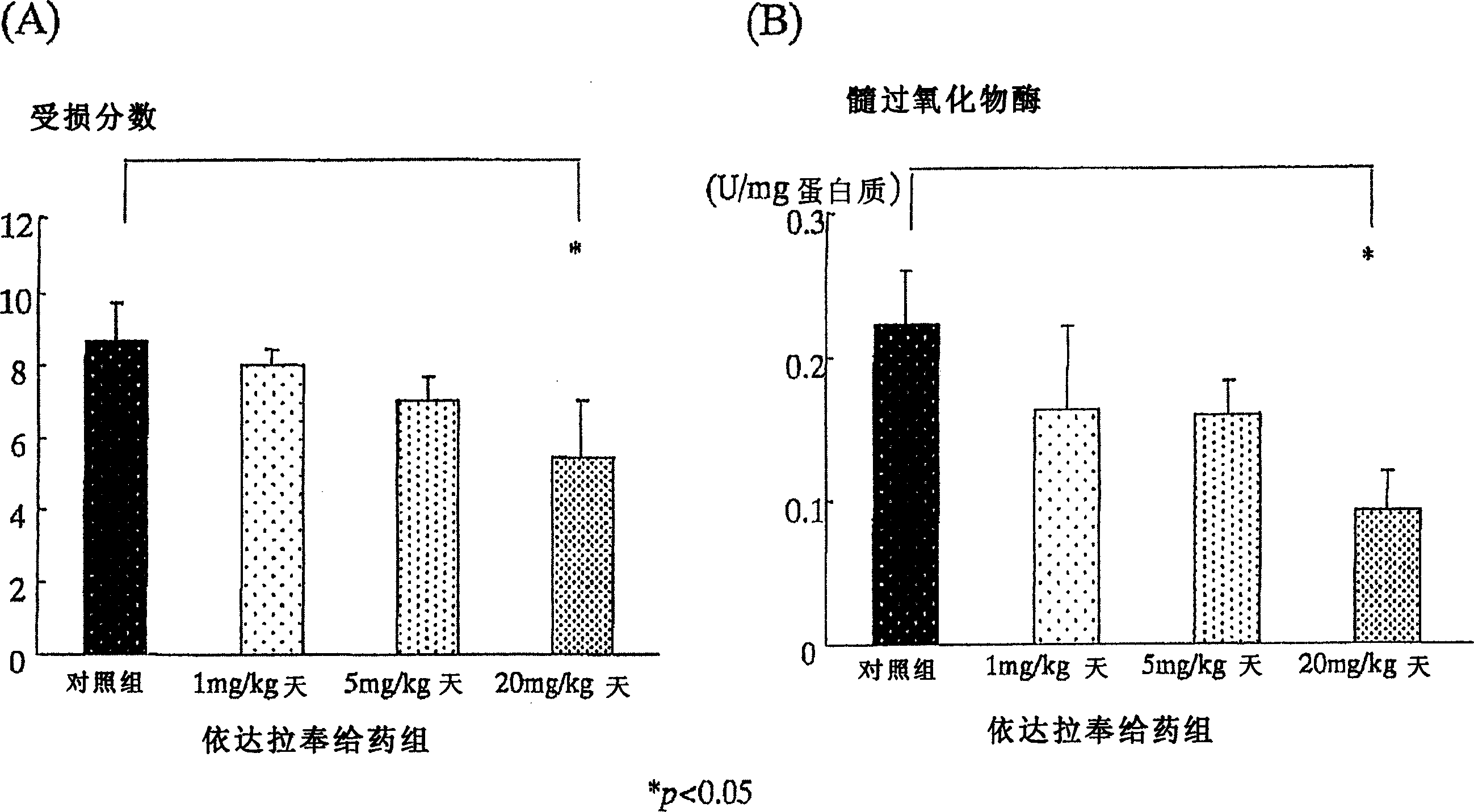
[0127] Using 6-week-old Sprague-Dawley male rats (Japan CLEA Co., Ltd., according to the existing method (Araki, Y., et al., Scand J Gastroentero, 35, 1060-1067, 2000), the following induced in rats Colitis. That is, add 4% (w / w) sugar anhydroester (DSS: molecular weight 5000, Wako Pure Chemical Industry Co., Ltd., Osaka) in powdered food MF (Oriental Yeast Industry Co., Ltd.), with 30g / The amount of day / only was fed to the rats. Since the beginning of DSS administration, the Edaravone administration group was injected with 1mg / kg / day, 5mg / kg / day, and 20mg / kg / day of Edaravone in the morning and evening respectively. Subcutaneously in the back. The control group also injected saline subcutaneously in the back. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com