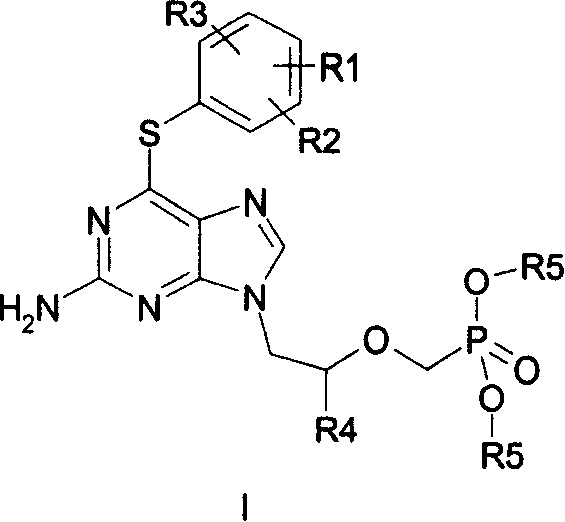
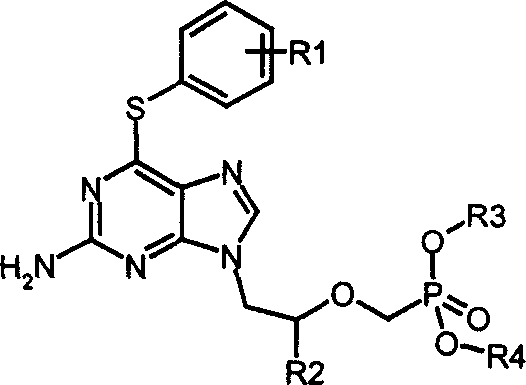
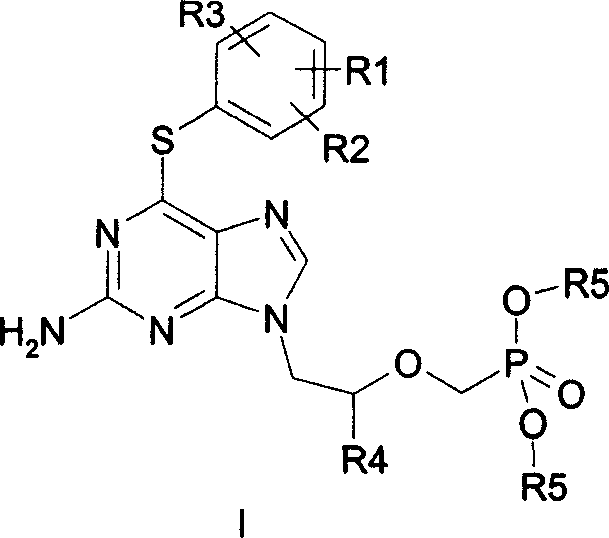
Acyclic nucleoside phosphonate derivatives
A technology of nucleoside phosphonate and derivatives, which is applied in the field of anti-hepatitis B virus, and can solve the problem that there are only a handful of anti-hepatitis B virus drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example
[0024] Reference example 2-amino-6-(4-methoxyphenylthio)-9-[2-[bis(2,2,2-trifluoroethyl)phosphonomethoxy]propyl]-purine ( Synthesis of MCC-478)
[0025] Synthetic MCC-478 was used as a control substance for biological evaluation.
[0026] 1. 2-[bis(2,2,2-trifluoroethyl)-phosphonomethoxy]-ethyl chloride
[0027] In 46 grams of trifluoroethanol, 21 grams of phosphorus trichloride was added, and the mixture was stirred and reacted at 80-90° C. for 4 hours. Fractional distillation yielded 41 grams of tris-(2,2,2-trifluoroethyl)phosphite, 130-131°C / 74-78mmHg.
[0028] Suspend 26 grams of 2-chloroethanol and 10 grams of paraformaldehyde in 50 ml of dichloromethane, cool in an ice bath; under stirring, dry hydrogen chloride at 0°C for 10 hours, separate the water layer, and chlorinate the organic layer with anhydrous Calcium drying, filtration to remove solids and distillation to obtain 24 grams of chloroethyl chloromethyl ether, boiling point 78-82 ° C / 30 mmHg
[0029] 18 g of...
Embodiment 1
[0034]Example 1 2-amino-6-(3,4-dimethoxyphenylthio)-9-[2-[bis(2,2,2-trifluoroethyl)phosphonomethoxy]ethyl ]-purine (I 1 )
[0035] 1.1 3,4-Dimethoxybenzenethiol
[0036] Under mechanical stirring, 2.64 g of o-methoxyanisole was slowly added in batches to 5 g of chlorosulfonic acid within 20 minutes. After stirring for 20 minutes, the reaction mixture was poured into 40 ml of ice water. After the ice had completely melted, it was extracted twice with dichloromethane, 15 ml each time. The extracts were combined and dried over anhydrous sodium sulfate. The desiccant was filtered off, and the filtrate was evaporated to dryness under reduced pressure to obtain a white solid, which was washed with a small amount of ether to obtain 3.3 g of 3,4-dimethoxybenzenesulfonyl chloride, melting point: 72-73°C.
[0037] Add 3.3 grams of 3,4-dimethoxybenzenesulfonyl chloride to 90 ml of ice water, add 5.5 ml of concentrated sulfuric acid, stir vigorously, add 5 grams of Zn powder in batche...
Embodiment 2
[0040] Example 2 2-amino-6-(2,4-dimethoxyphenylthio)-9-[2-[bis(2,2,2-trifluoroethyl)phosphonomethoxy]ethyl ]-purine (I 2 )
[0041] 2.1 2,4-Dimethoxybenzenethiol
[0042] Under full stirring, 29 grams of concentrated sulfuric acid was added dropwise to 27 grams of 1,3-dimethoxybenzene, and the addition was completed within 15 minutes. Stirring was continued for 1 hour, and slowly poured into 250 ml of saturated potassium carbonate aqueous solution. Filter the precipitate, dry it at 125°C, grind it finely, take 30 grams and add it to 35 grams of phosphorus oxychloride. The mixture was reacted in a steam bath for 2 hours. Cool to room temperature and pour into 300 ml of crushed ice. After the ice had completely melted, it was extracted twice with 150 ml of ether. The extracts were combined, washed with saturated brine, dried and evaporated to dryness under reduced pressure, and the obtained solid was washed with ether to obtain 18 g of 2,4-dimethoxybenzenesulfonyl chloride...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com