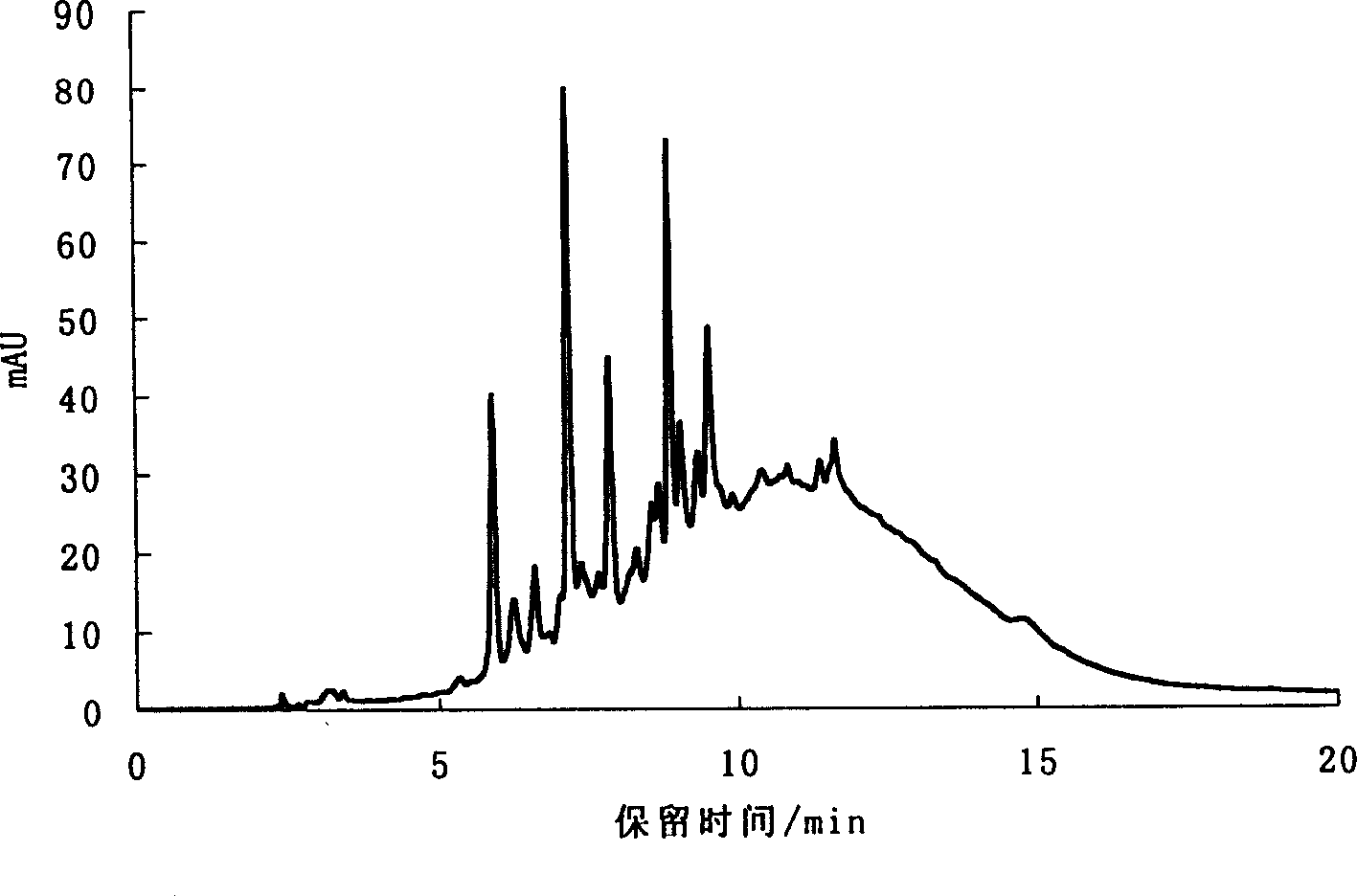
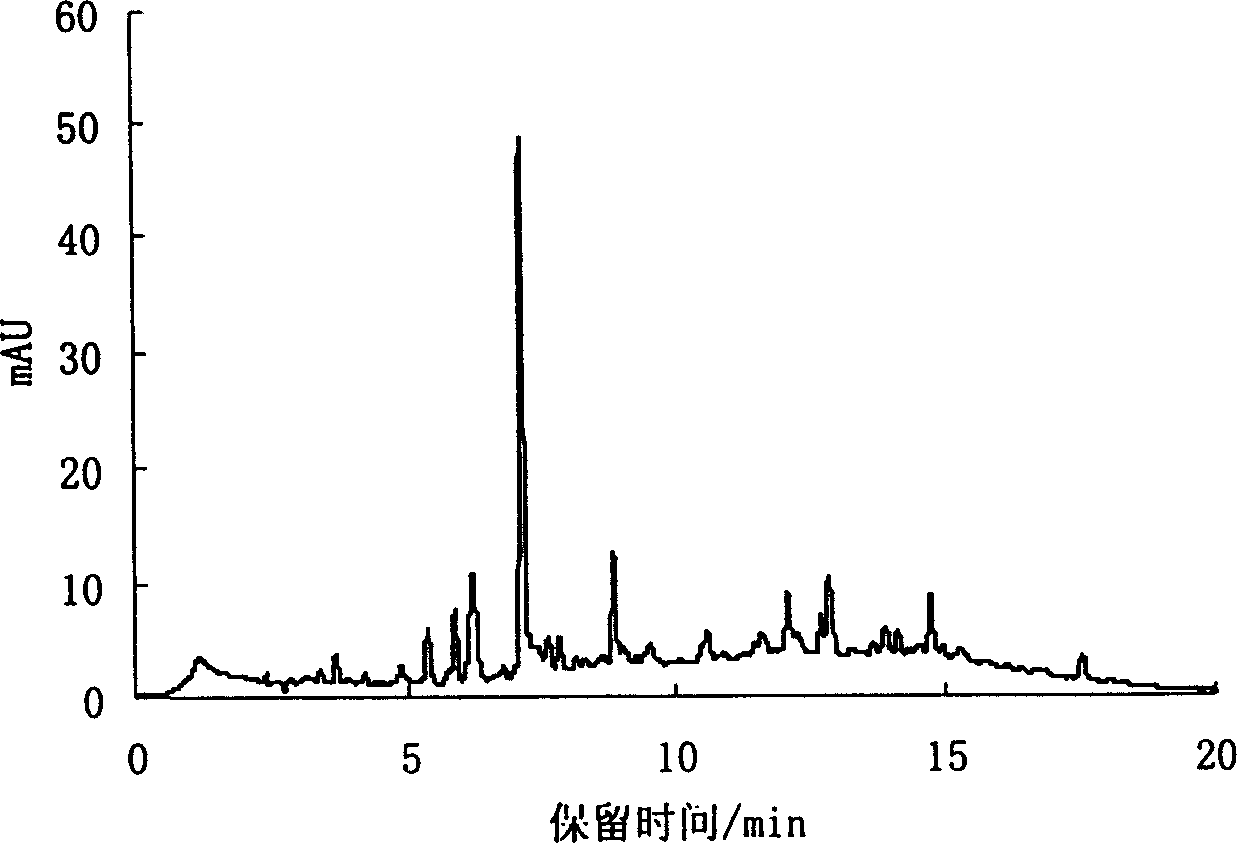
Preparation of oligomer and monomer from tannin by catalytic hydrogen degradation
A technology for plant tannin and catalytic hydrogenolysis, which is applied in the preparation of organic compounds, organic chemistry methods, chemical instruments and methods, etc. Problems such as hydroxyl oxidation, loss of biological and chemical activity of products, etc., to achieve the effect of improving resource utilization and added value, less waste discharge, and high product value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of oligomeric proanthocyanidins by extraction, fractionation and catalytic hydrogenolysis of larch proanthocyanidins
[0034] The first step: Larix proanthocyanidin extraction and classification
[0035]Air-dried larch bark is used as raw material, crushed into particles with a particle size of 1-15mm, and sieved to remove impurities. Then, weigh 10 kg of the above-mentioned materials, add 100 L of 70% volume ratio ethanol aqueous solution, and extract in an extraction tank at a temperature of 70±5°C for 4 hours under forced reflux with a pump, and then discharge. Remove the slag through coarse filtration, pump the resulting filtrate into a vacuum concentration tank, reclaim ethanol, discharge, and then filter, and the precipitated part is dried to obtain 736g reddish-brown powder, which is proanthocyanidin high polymer (LPE); in the filtrate Add petroleum ether or light oil, oscillate or stir, and let stand to separate layers to remove esters an...
Embodiment 2
[0038] Example 2: Preparation of oligomeric proanthocyanidins from larch extract by catalytic hydrogenolysis
[0039] Take 200g of larch extract (not treated with sulfurous acid), add 5g of Pd-C catalyst, add 1000ml of ethanol with a volume ratio of 55%, mix well, put it into an autoclave, feed hydrogen to make the air pressure 0.2MPa, and The temperature was 60°C, and the reaction was carried out for 4 hours under stirring. Stop heating, and when the temperature drops to room temperature, exhaust to normal pressure first, and take out the reaction product. Filter, concentrate under reduced pressure, and dry to obtain 135 g of the product (LPH). The obtained product was dissolved in water, and then extracted four times with ethyl acetate to obtain the extract layer. After drying, 70.7 g of reddish-brown oligomeric proanthocyanidin (LPHC) was obtained, accounting for about 52.37%; while the water layer of the raffinate phase was brown poly-proanthocyanidin (LPHW), weighing 26...
Embodiment 3
[0040] Example 3: Preparation of ellagic acid by catalytic hydrogenolysis of oak extracts
[0041] Take 100g of extract in a rubber bowl, add 2g of palladium-carbon catalyst, add 800ml of ethanol with a volume ratio of 45%, mix well, put it into an autoclave, feed hydrogen gas to make the pressure 10MPa, and react 4 at a temperature of 80°C under stirring. Hour. Stop heating, when the temperature drops to room temperature, exhaust to normal pressure first, take out the reaction product, heat to above 70°C, filter while hot to remove the residue, concentrate the filtrate under reduced pressure, cool, filter with suction, and wash the filter cake with distilled water 2 to 3 times and discard the filtrate. Then the filter cake was washed with pyridine to dissolve ellagic acid, and after several recrystallizations, 13.2 g of light yellow ellagic acid crystals were obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com