Controlled release preparation for nitrendipine of constant speed release
A technology for nitren and preparations, applied in the field of controlled release preparations, can solve problems such as severe blood pressure fluctuations, high T/P ratio, changes in blood drug concentration, etc. smooth effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0008] Specific embodiment, medicine preparation: (mg / tablet)
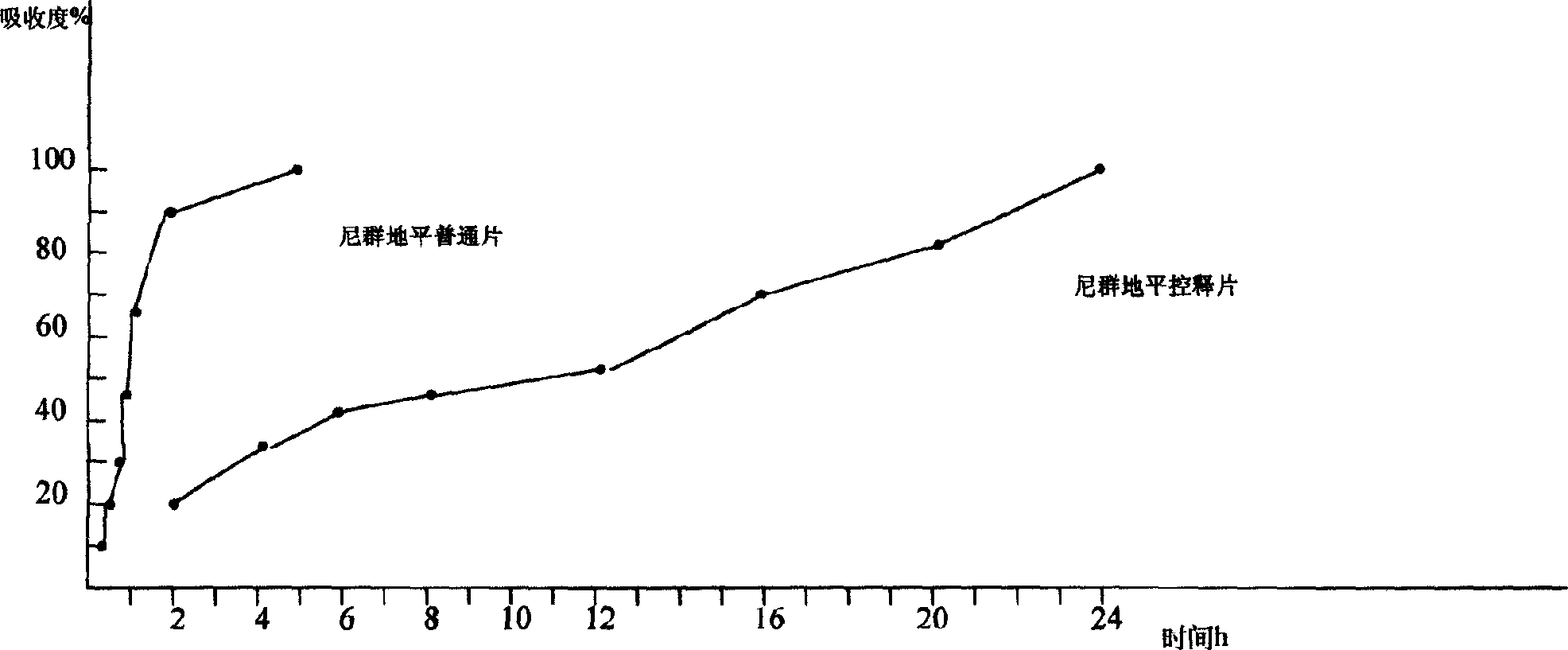
[0009] Nitrendipine 30, hydroxypropyl methylcellulose 40, calcium hydrogen phosphate 30, sodium alginate 20, magnesium stearate 1% (dry grain weight), appropriate amount of solvent and other auxiliary materials. According to the prepared formula, each raw material was crushed through a 20-mesh sieve, wet granulated, added a solvent to form a ball, loosened after loosening, dried, sieved and granulated, added magnesium stearate, mixed and pressed into tablets. have to. And experiment among Fig. 1, according to Pharmacopoeia in vitro release assay method, survey product in vitro release of the present invention, stripping medium is the artificial gastric juice containing ethanol, takes a sample within the prescribed time, filters and measures absorbance at 238nm. It is found that the nitrendipine controlled-release tablets prepared according to the present invention can basically release at a constant rate and mai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com