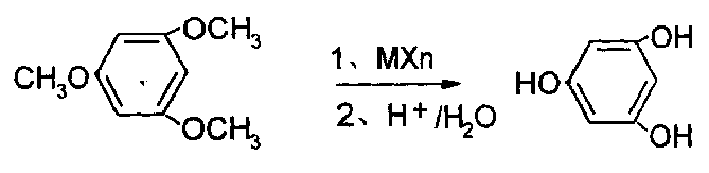Preparation method of resorcinol
A technology of phloroglucinol and trimethoxybenzene, which is applied in the field of preparation of phloroglucinol, can solve problems such as post-processing difficulties, and achieve the effects of easy implementation, high product purity, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The preparation method of phloroglucinol of the present invention is to take 1,3,5-trimethoxybenzene and Lewis acid as raw materials, react in hydrocarbons, halogenated hydrocarbons, aromatic hydrocarbons or substituted aromatic hydrocarbon solvents, and react The temperature is 40-150°C, and then acidic hydrolysis with dilute acid is added. After the acidic hydrolysis is completed, the layers are separated, the water layer is cooled and crystallized, and then recrystallized to obtain phloroglucinol with a content of more than 98.0%.
[0013] The solvent used for the reaction is: hydrocarbons, halogenated hydrocarbons, aromatic hydrocarbons or substituted aromatic hydrocarbon compounds. Hydrocarbons refer to: solvent gasoline, petroleum ether, hexane, cyclohexane, heptane, halogenated hydrocarbons refer to: dichloromethane, chloroform, carbon tetrachloride, dichloroethane, aromatic hydrocarbons or substituted Aromatics refer to: benzene, toluene, xylene, chlorobenzene, ...
Embodiment 1
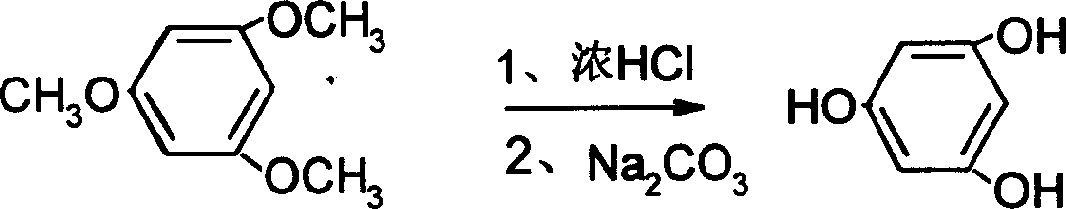
[0020] Add 300mL of chlorobenzene to a 1000mL three-necked flask equipped with a reflux condenser, dropping funnel, and stirrer, add 0.2mol of trimethoxybenzene and 95.2g (0.7mol) of zinc chloride one by one under stirring, and heat to 120°C. When an intermediate had formed, the reaction was continued for 1 hour and cooled to room temperature. Add dropwise 500mL of 2NHCl acidolysis intermediate therein, after acidolysis is complete, separate layers, cool and crystallize the water layer, and filter to obtain crude phloroglucinol, which is recrystallized with water and dried to obtain 22.8g of phloroglucinol finished product (content 98.7% ), yield 89.3%.
Embodiment 2~ Embodiment 9
[0022] Change the type or amount of Lewis acid, others are the same as in Example 1, and the results are shown in Table 1
[0023] Example
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com