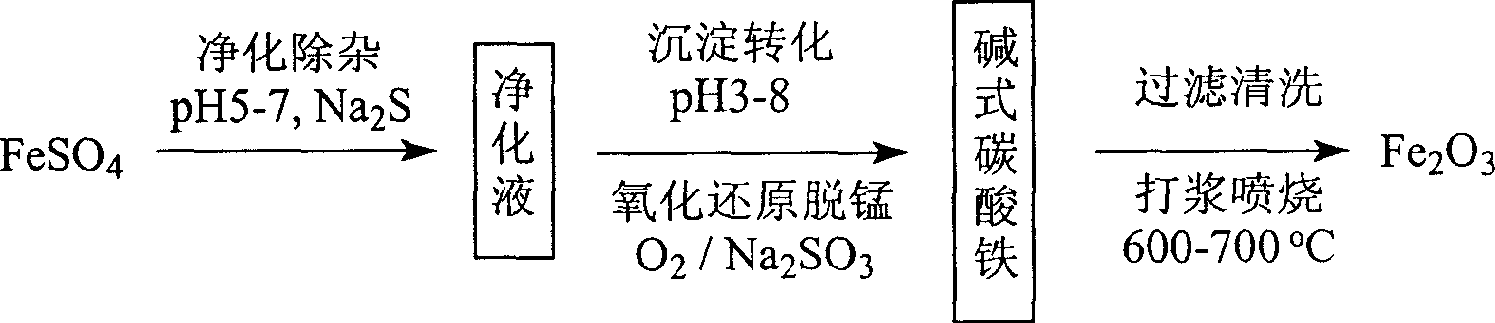
Process for preparing ferric oxide red powder
An iron oxide and red powder technology, applied in fibrous fillers and other directions, can solve the problems of inability to enter the mid-to-high-end market, insufficient utilization of ferrous sulfate by-products, and excessive manganese content. It is suitable for large-scale industrial production and easy to control. , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 1.9kg of industrial ferrous sulfate into a 5L beaker equipped with a mechanical stirrer, add deionized water to 4.0L, and add 30g of iron sheet to consume free acid in the raw materials. Maintained at 80-90°C and stirred for 4 hours. After removing the residual iron sheet, add 0.8g of sodium sulfide, 50mL of 4.3% ammonia water under the condition of pH=5, blow in air for 30 minutes, keep warm for 0.5 hours, add 30mL of 4.3% ammonia water, add 0.1g of polyacrylamide, and continue to keep warm After 4 hours let stand overnight. The filtrate was filtered through a 0.7 μ filter membrane to obtain a clear and transparent green filtrate. After analysis of the filtrate, Fe57.24g / L, Mn676mg / L, Si<0.5mg / L, Al<0.1mg / L, Ca2.6mg / L. Yield 78.5%.
Embodiment 2
[0022] Xiangyi 3M 3 Inject 1.5M into the reactor 3 Put 900kg of industrial ferrous sulfate into deionized water, add 1.5kg of sodium sulfide and 250 liters of 4.4% ammonia water under stirring, heat up to 80-90°C at the same time, blow in air for 30 minutes, add 60g of polyacrylamide, and keep warm for 5 After hours let stand overnight. The supernatant was filtered through a 0.5μ microporous filter to obtain a clear and transparent green filtrate 2.0M 3 . After analysis of the filtrate, Fe67.9g / L, Mn660mg / L, Si1.3mg / L, Al0.35mg / L, Ca6.2mg / L, Mg285mg / L. Yield 85%.
Embodiment 3
[0024] Fe 2 o 3 (%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com