Controlled release formulation of divalproex sodium
A technology of divalproex sodium and valproic acid, which is applied in the fields of medical preparations of non-active ingredients, pill delivery, active ingredients of anhydrides/acids/halides, etc., and can solve problems such as inappropriate dosing regimens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
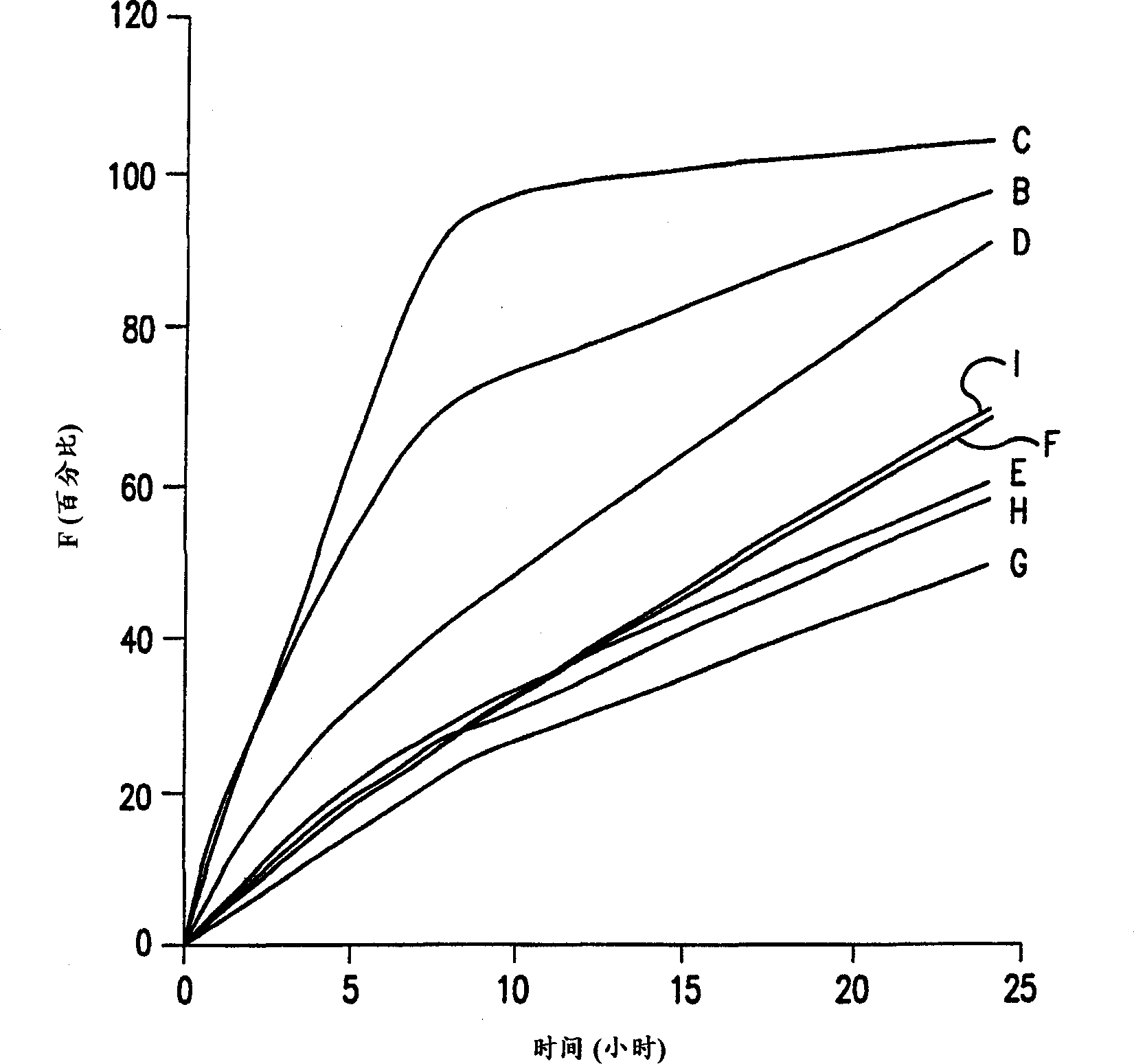
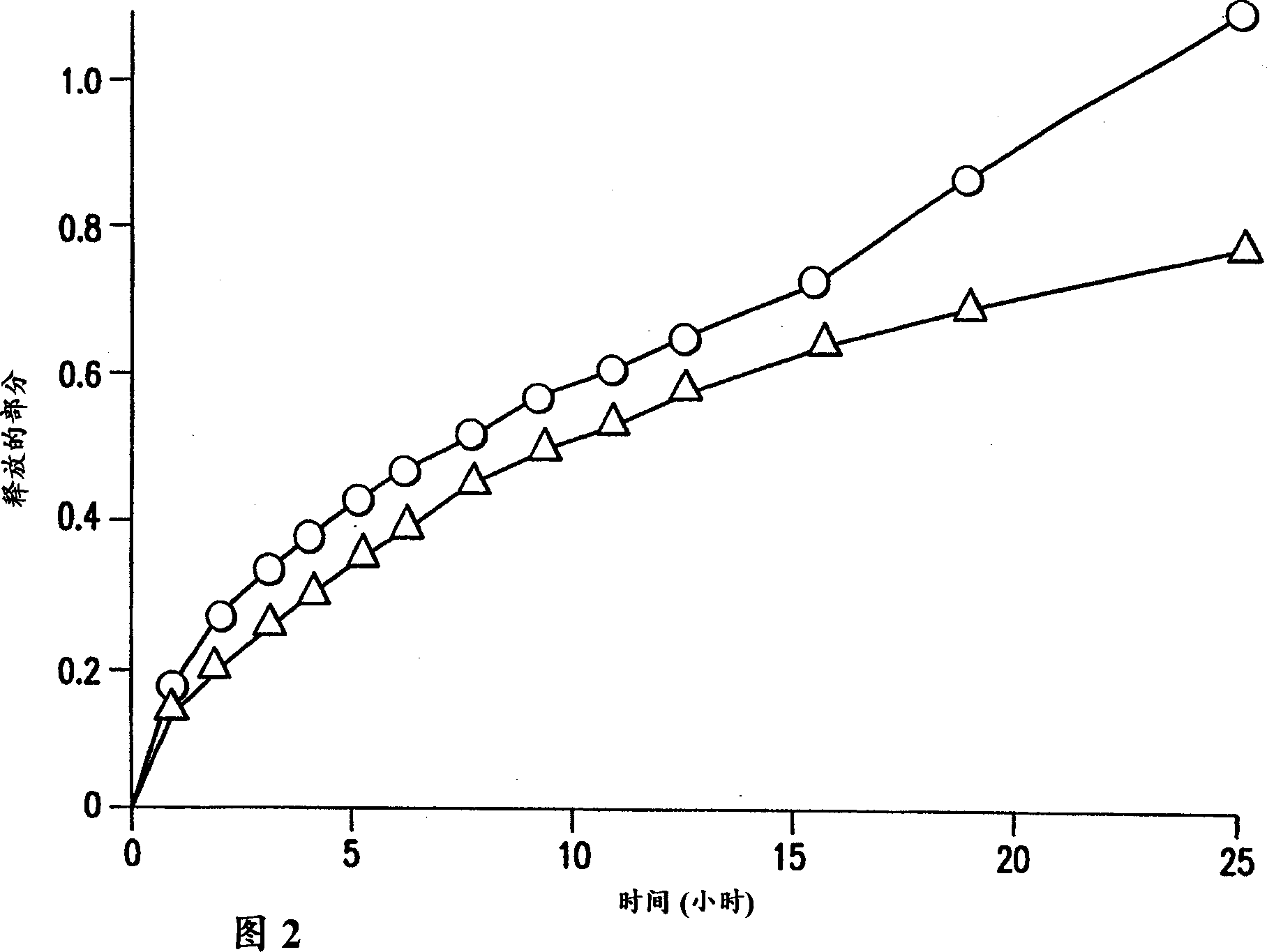
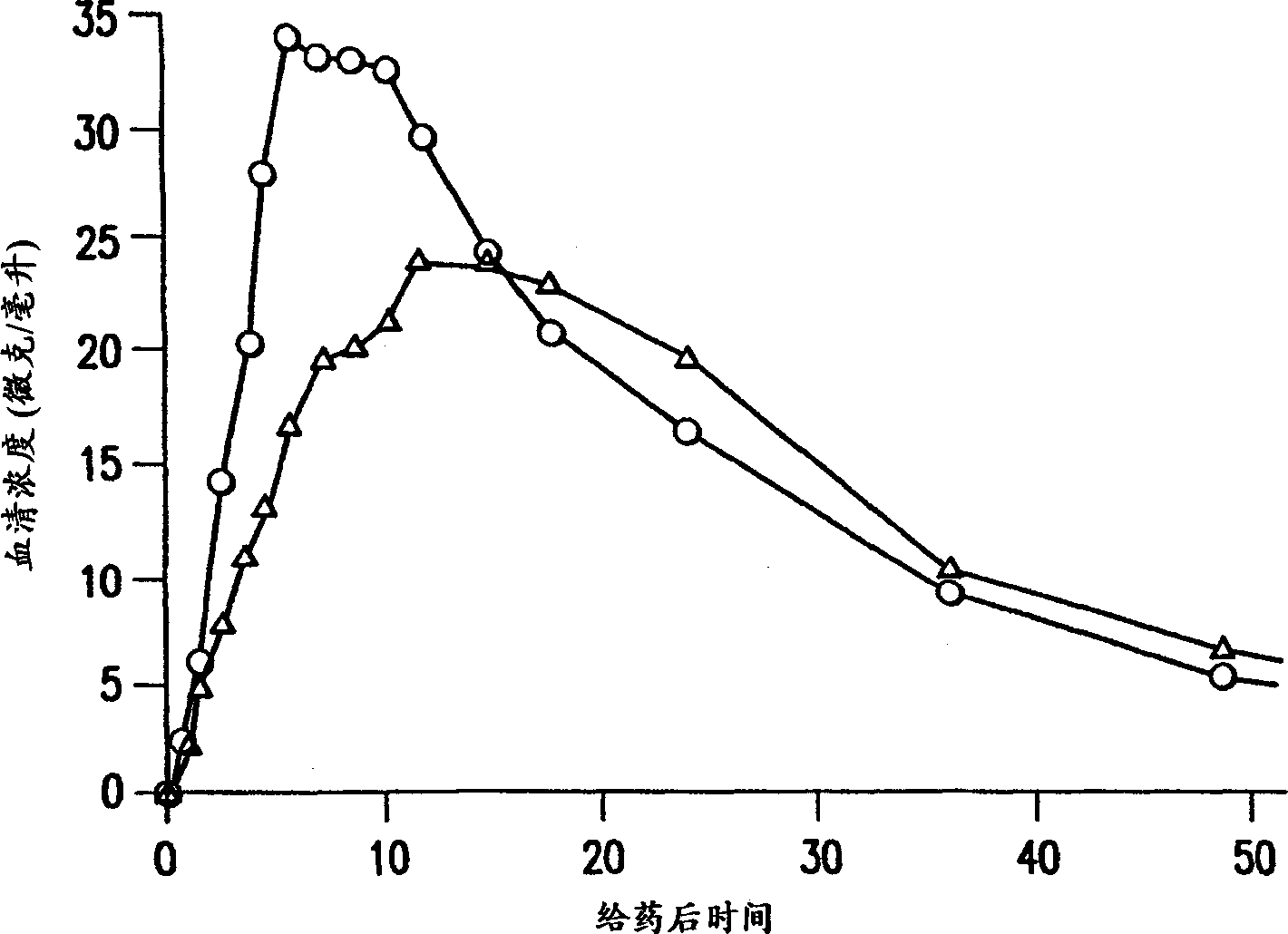
[0022] The terms "sustained release", "delayed release" and "controlled release" as applied to pharmaceutical formulations in the specification and claims of this application are defined in "Remington's Pharmaceutical Sciences," 18 th Ed., p. 1677, Mack Pub. Co., Easton, PA (1990) on their meaning. Sustained-release drug systems include any drug delivery system that achieves sustained release of a drug over an extended period of time, and includes time-release and controlled-release systems. Such a sustained release system is considered a controlled release drug delivery system if it is effective in maintaining substantially constant drug levels in the blood and target tissues. However, if a drug delivery system fails to achieve substantially constant blood or tissue drug levels, but prolongs the duration of drug action beyond that achieved by conventional release, it is considered a delayed release system.
[0023] The formulations of the present invention provide controlled...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com