Zero level drug administration oral administration controlled-release tablet and preparation thereof
A technology for controlled-release tablets and drug delivery, which is applied in pharmaceutical formulations, devices for making drugs into special physical or taking forms, and drug delivery. It can solve problems such as insufficient research and development, and achieve good drug release reproducibility. The effect of high degree of automation and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the deployment of layer powder and binder
[0035] Pass 5 cps ethyl cellulose through a 200-mesh sieve, and take powder with a particle size of less than 74 μm for the top and bottom layers; weigh 4 grams of 5 cps ethyl cellulose powder, dissolve it in 100 mL of 90% ethanol aqueous solution, and prepare into top and bottom powder forming binders.
[0036] The raw material composition and content (by weight percentage) of intermediate mixing powder are as follows:
[0037] Hydroxypropyl methylcellulose HPMC E50 35 parts
[0038] Lactose 54 parts
[0039] Polyvinylpyrrolidone K30 10 parts
[0040] 1 part colloidal silicon dioxide
[0041] Take by weighing 5 grams of polyvinylpyrrolidone K30 powder and be dissolved in 100mL of 75% aqueous ethanol to be prepared as a drug-free binder in the middle zone; take by weighing 12 grams of diclofenac sodium powder, be dissolved in 100mL of 75% aqueous ethanol,
Embodiment 2
[0042] Example 2: Determining 3D printing forming parameters
[0043] Top and bottom surface spray forming parameters:
[0044] Layer interval time 3min
[0045] Powder layer thickness 200μm
[0046] Spraying rate [spraying drop volume (droplet quantity × droplet size) × spraying frequency] 0.4nL × 12kz
[0047] Spray times 3 times
[0048] The spray forming parameters of the fully sprayed drug layer in the middle drug-loading area:
[0049] Layer interval time 2min
[0050] Powder layer thickness 200μm
[0051] Spray rate (spray drop volume × spray frequency) 0.4nL × 12kz
[0052] Spraying times 2 times
[0053] Spraying forming parameters of the intermediate drug-loading area partly sprayed with drug-containing binder and partly sprayed with drug-free binder:
[0054] Layer interval time 4min
[0055] Powder layer thickness 200μm
[0056] Spray rate (spray drop volume × spray frequency) 0.4nL × 12kz
[0057] Spraying times (medicated binder and some non-medicated bi...
Embodiment 3
[0058] Embodiment 3: Optimization of spray drop spacing
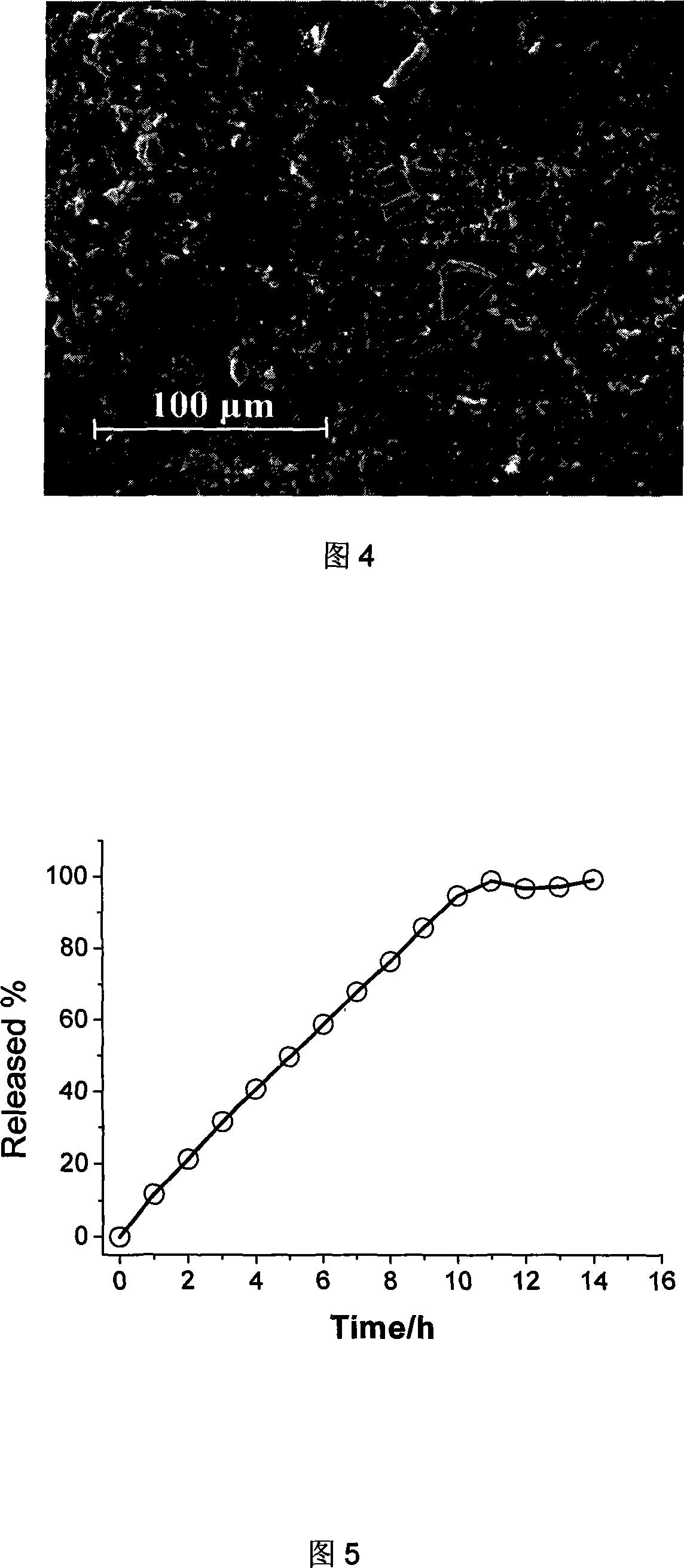
[0059] Printing and spraying tests with different drop spacings were carried out on the intermediate mixed powder with a thickness of 200 μm. The bonding effect and the migration and spread of printing liquid powders were observed and compared through three-dimensional video microscopic controlled release tablets, and the most suitable drop spacing was optimized. .
[0060] As shown in Figure 3, when the droplet distance is 50 μm, it can be seen that there is a better bonding effect, and the size of the droplet is 40 μm, allowing the adhesive to migrate and spread at 10 μm is more conducive to the uniformity of the drug and the bonding effect. The white filamentous substance is incompletely dissolved hydroxypropyl methylcellulose.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com