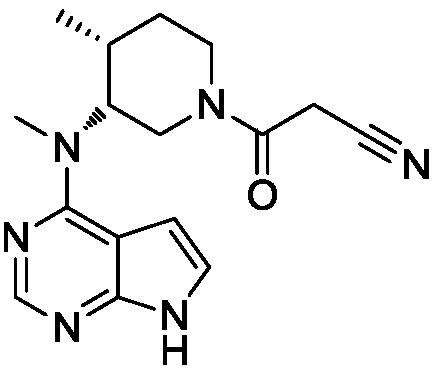
Tofacitinib controlled release tablet, preparation method and application of Tofacitinib controlled release tablet
A technology of tofacitinib and controlled-release tablets, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of inconvenient operation, long production cycle, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
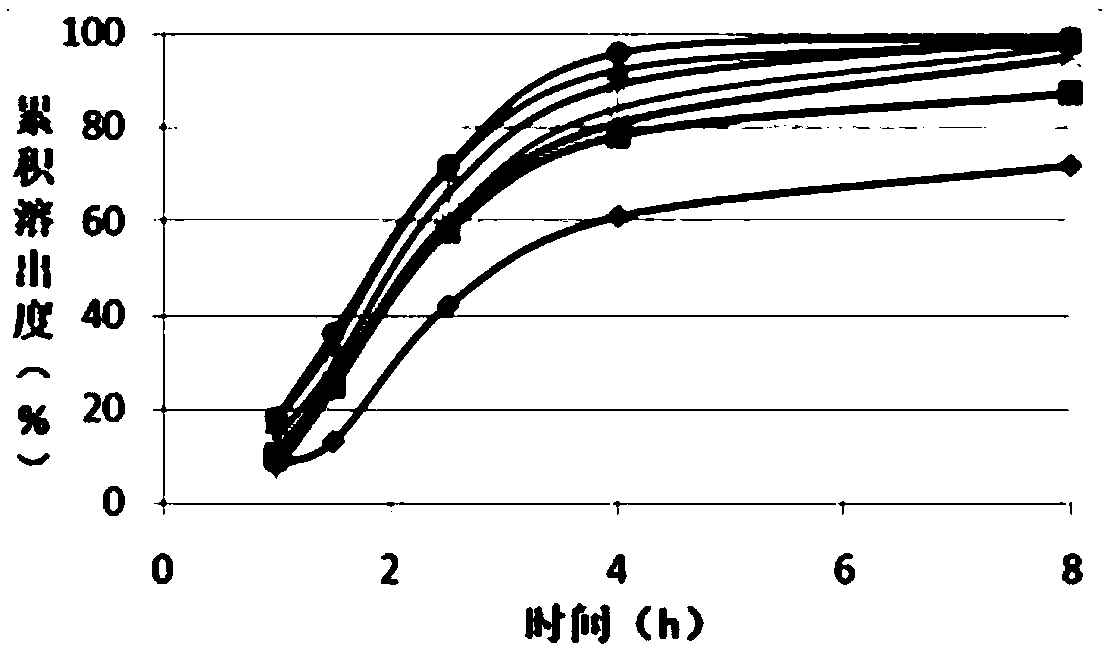
Image
Examples
Embodiment 1
[0067] Tablet core prescription
[0068] name Mass percentage (%) Tofacitinib citrate 8.9 lactose monohydrate 66.1 Crospovidone 12 Hydroxypropyl Cellulose 6 Copovidone 6 Magnesium stearate 1
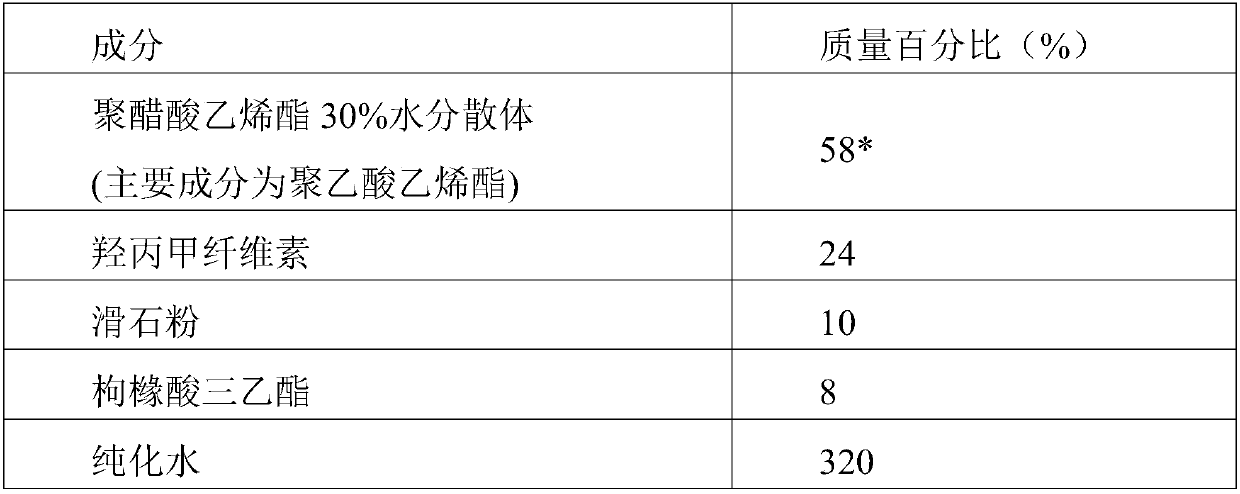
[0069] Coating prescription
[0070]
[0071] * is the solid content of polyvinyl acetate 30% aqueous dispersion.
[0072] Step (1) Premixing: Weigh the prescribed amount of tofacitinib citrate, lactose monohydrate, crospovidone, hydroxypropyl cellulose, and copovidone in a three-dimensional mixer, and set the frequency to 30Hz for mixing 10 minutes to obtain a mixture. The obtained mixture was passed through a 30-mesh sieve, placed in a three-dimensional mixer, and mixed for 5 minutes to obtain a premix.
[0073] Step (2) total blending: the premix obtained in step (1) is added with a prescribed amount of magnesium stearate, added to a three-dimensional mixer, set at a frequency of 30 Hz, and mixed for 5 minutes to obtain a total...
Embodiment 2
[0079] Tablet core prescription
[0080] name Mass percentage (%) Tofacitinib citrate 8.9 lactose monohydrate 68.1 Crospovidone 8 Hydroxypropyl Cellulose 8 Copovidone 6 Magnesium stearate 1
[0081] Coating prescription
[0082]
[0083]
[0084] * is the solid content of polyvinyl acetate 30% aqueous dispersion.
[0085] Step (1) Premixing: Weigh the prescribed amount of tofacitinib citrate, lactose monohydrate, crospovidone, hydroxypropyl cellulose, and copovidone in a three-dimensional mixer, and set the frequency to 30Hz for mixing 10 minutes to obtain a mixture. The obtained mixture was passed through a 30-mesh sieve, placed in a three-dimensional mixer, and mixed for 5 minutes to obtain a premix.
[0086] Step (2) total blending: add the prescribed amount of magnesium stearate to the premix obtained in step (1), add it into a three-dimensional mixer, set the frequency to 30 Hz, and mix for 5 minutes to obtain a...
Embodiment 3
[0092] Tablet core prescription
[0093] name Mass percentage (%) Tofacitinib citrate 8.9 lactose monohydrate 70.1 Crospovidone 6 Hydroxypropyl Cellulose 8 Copovidone 6 Magnesium stearate 1
[0094] Coating prescription
[0095]
[0096] * is the solid content of polyvinyl acetate 30% aqueous dispersion.
[0097] Step (1) Premixing: Weigh the prescribed amount of tofacitinib citrate, lactose monohydrate, crospovidone, hydroxypropyl cellulose, and copovidone in a three-dimensional mixer, and set the frequency to 30Hz for mixing 10 minutes to obtain a mixture. The obtained mixture was passed through a 30-mesh sieve, placed in a three-dimensional mixer, and mixed for 5 minutes to obtain a premix.
[0098] Step (2) total blending: add the prescribed amount of magnesium stearate to the premix obtained in step (1), add it to a three-dimensional mixer, set the frequency to 30 Hz, and mix for 5 minutes to obtain the total blend; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com