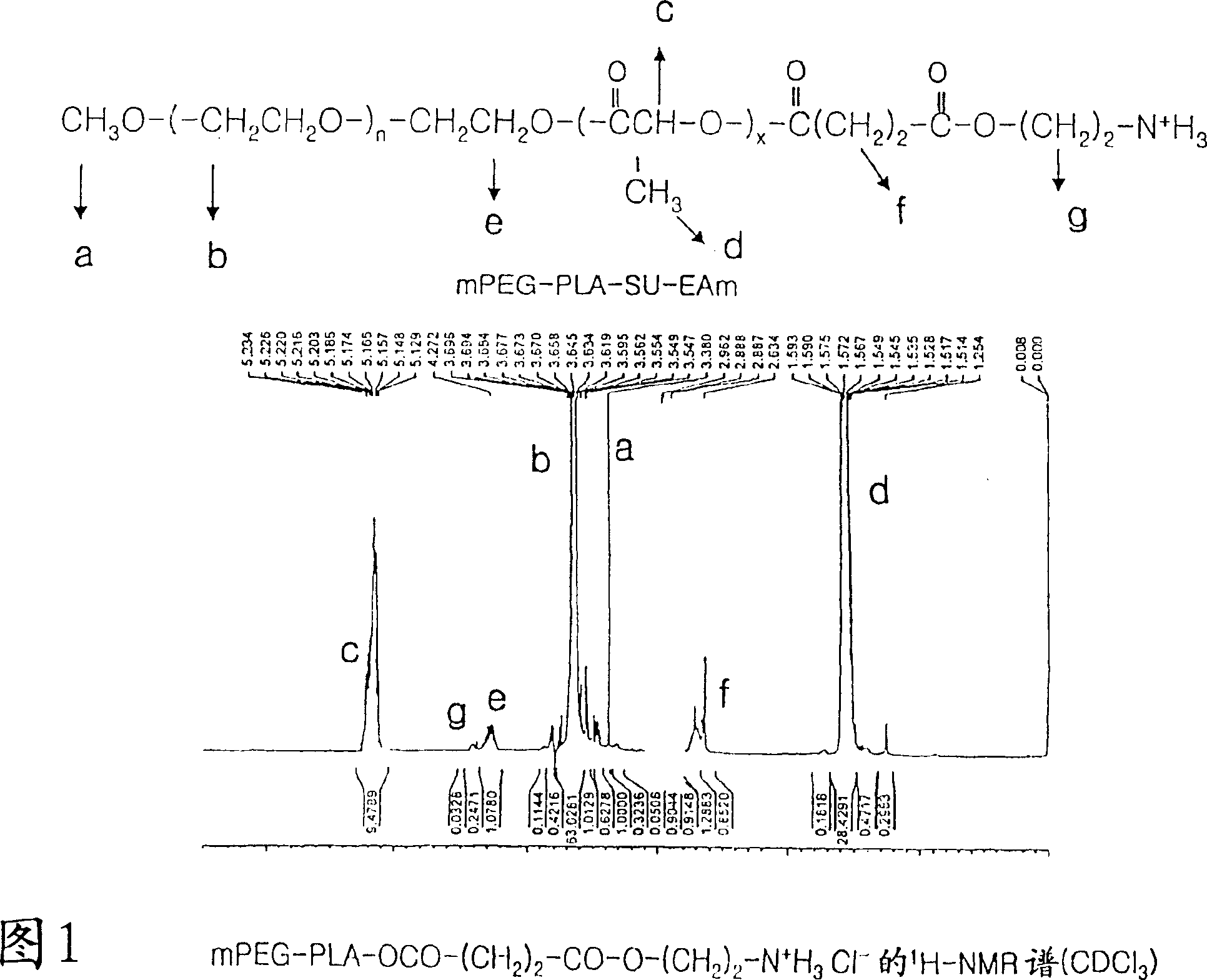
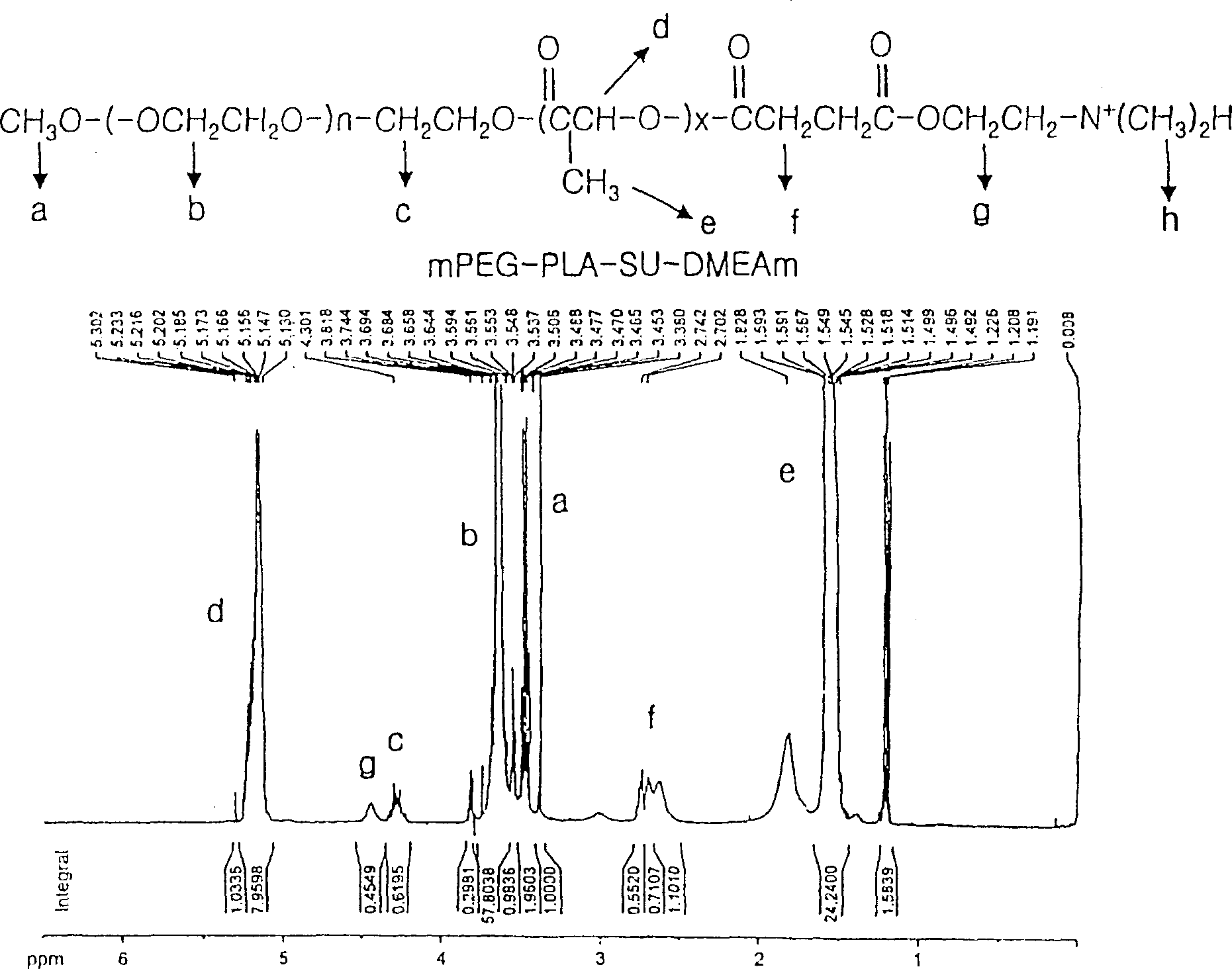
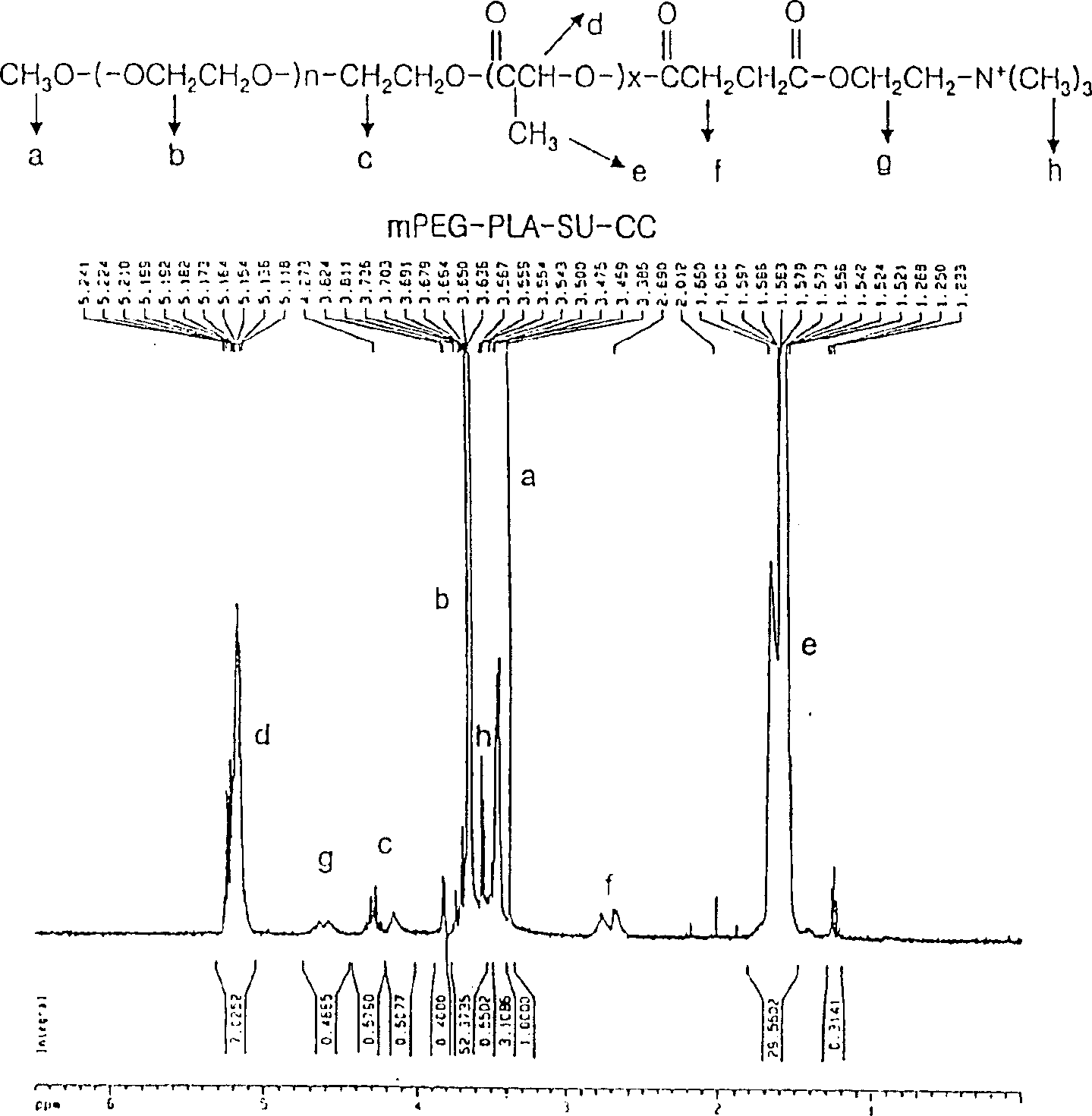
Positively charged amphiphilic block copolymer as drug carrier and complex thereof with negatively charged drug
A technology of block copolymers and hydrophilic polymers, which is applied in the field of transporting anionic bioactive reagents and polymer micellar drug carriers, and can solve the problems of reducing drug stability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Example 1: Amino-containing (-O-C(=O)CHR 1 -NH 3 + Cl - ) Synthesis of methoxypolyethylene glycol-polylactide (mPEG-PLA-O-C(=O)CHR 1 -NH 3 + -Cl - )
[0121] The block copolymer prepared in Preparation 1 and having an -OH group at the chain end of the PLA (B above) block was reacted with an amino acid derivative having a protecting group on the amino group to obtain the title block copolymer.
[0122]
[0123] Dissolve 0.583 g of N-benzyloxycarbonyl-p-hydroxyphenylglycine, 0.575 g of dicyclohexylcarbodiimide (DCC) and 7.0 g of methoxypolyethylene glycol-polylactide (mPEG-PLA, 2000-1765) in 20ml DMF. The resulting solution was reacted at room temperature for 24 hours to obtain N-benzyloxycarbonyl-p-hydroxyphenylglycine methoxypolyethylene glycol-polylactide. Using palladium as a catalyst, the reaction product was hydrogenated to remove the protecting group on the amino group, dissolved in aqueous hydrochloric acid, dialyzed, and then lyophilized to obtain...
Embodiment 2-4
[0124] Embodiment 2-4: the introduction of amino acid group
[0125] In the same manner as in Example 1, each block copolymer prepared in Preparations 2-4 was reacted with an amino acid derivative having a protecting group on the amino group to obtain a copolymer containing an amino acid group. Table 2 below shows the amino acid group-containing block copolymers prepared in Examples 1-4.
[0126] Example
Embodiment 5
[0127] Embodiment 5: Contain aminoethanol (-O-CH 2 CH 2 N + R 3 -Cl - ) Synthesis of methoxypolyethylene glycol-polylactide (mPEG-PLA-O-C(=O)(CH 2 ) Z C(=O)-O-CH 2 CH 2 N + R 3 -Cl - )
[0128] The block copolymer prepared in Preparation 1 was reacted with the diacid chloride to give the carboxylic acid derivative, and then the reaction product was reacted with the 2-aminoethanol derivative to give the title block copolymer.
[0129]
[0130] (where z represents an integer from 0-6)
[0131] 7 g of methoxypolyethylene glycol-polylactide (mPEG-PLA, 2000-1765) and 5 g of excess succinyl dichloride were dissolved in chloroform, 1 ml of pyridine was added thereto, and the mixture was reacted at 60° C. for 12 Hour. The resulting solution was added to diethyl ether to precipitate a block copolymer. The precipitated block copolymer was dissolved in 10 ml of N-methylpyrrolidone, 0.363 g of ethanolamine hydrochloride was added thereto, and the mixture was reacted a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com