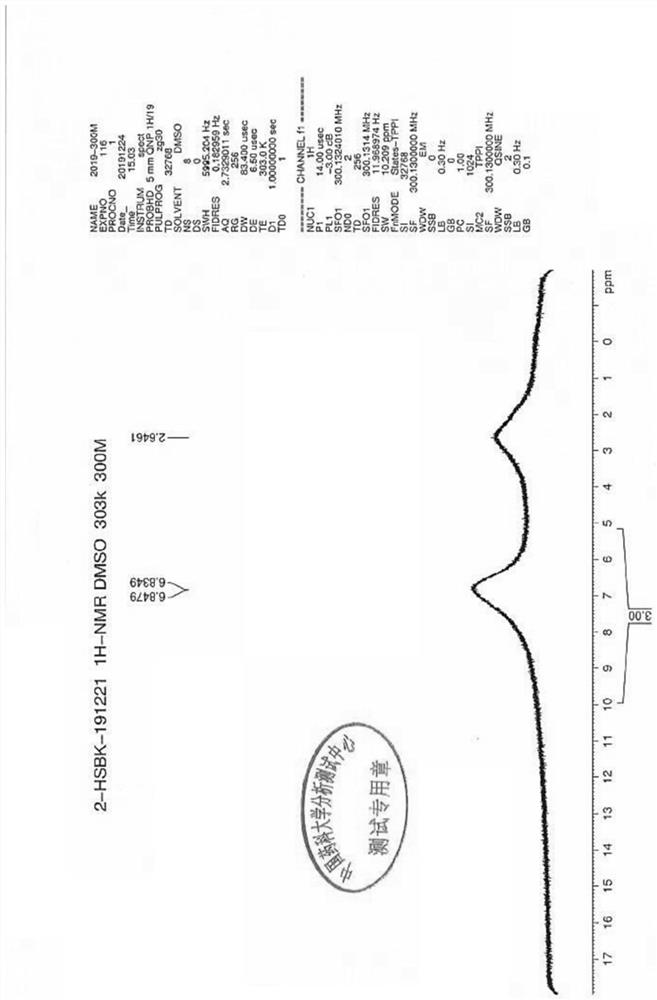
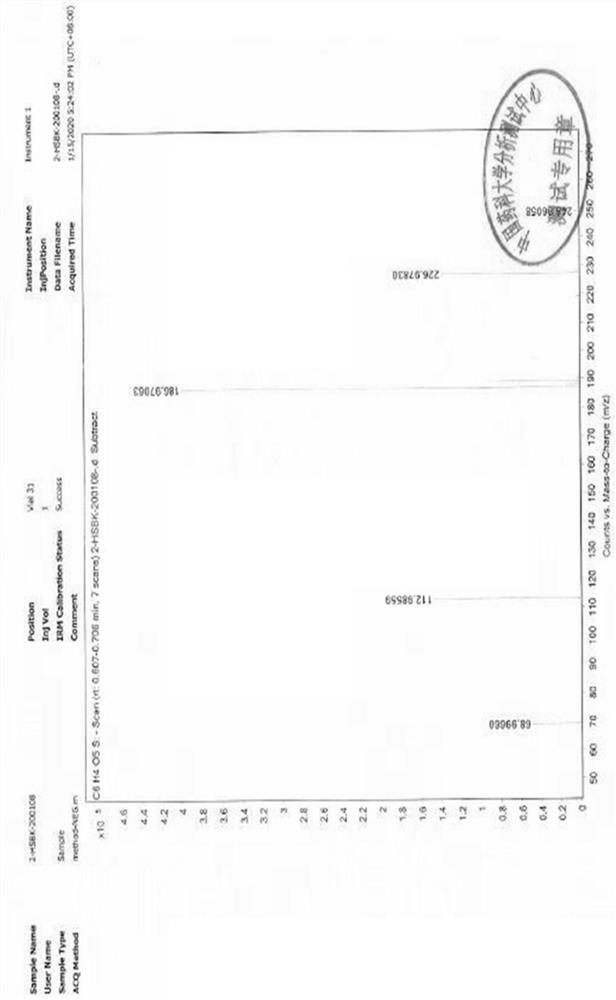
Quantitative determination method for genotoxic impurities in calcium dobesilate
A technology for quantitative determination of calcium dobesilate, applied in chemical instruments and methods, measuring devices, and other chemical processes, can solve the problems of increasing the difficulty of impurities, difficult to separate, and inability to coexist, so as to ensure quality controllability, Mild reaction conditions and good method specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Method verification for the determination of benzoquinone impurities 1,4-benzoquinone and 2-sulfo-1,4-benzoquinone in calcium dobesilate
[0060] Column: Phenomenex Gemini C 18 Chromatographic column (size 4.6mm×250mm, 5μm);
[0061] Mobile phase: 20mmol / L ammonium dihydrogen phosphate solution-acetonitrile (50:50);
[0062] Detection wavelength: 378nm;
[0063] Flow rate: 1.0mL / min;
[0064] Column temperature: 25°C.
[0065] 1.1 Specificity test
[0066] (1) Investigate the interference of blank solvent
[0067] Due to the high concentration of the derivatization reagent, the impurities in the derivatization reagent will appear peaks before and after the component to be measured, which will interfere with the sample determination. Therefore, it is stipulated that the separation between the impurities of the target component and the derivatization reagent should be greater than 2.0.
[0068] Take an appropriate amount of 1,4-benzoquinone reference subs...
Embodiment 2
[0095] Example 2 Determination of the content of 2-sulfo-1,4-benzoquinone and 1,4-benzoquinone in the calcium dobesilate tablet of the present invention.
[0096] Take an appropriate amount of 2-sulfo-1,4-benzoquinone manganese salt reference substance and 1,4-benzoquinone reference substance, add 50% acetonitrile to dissolve and prepare each 1ml containing 2-sulfo-1,4- A mixed solution of 1.4 μg each of benzoquinone and 1,4-benzoquinone, accurately measure 1ml, put it in a 10ml volumetric flask, add 6ml of derivatization solution, add water to dilute to the mark, shake well, react at room temperature for 5h, as a reference substance solution. Take an appropriate amount of calcium dobesilate tablet to be tested, put it in a 10ml volumetric flask, add 6ml of derivatization solution, react at room temperature for 5h, add water to dissolve and dilute to the mark, shake well, filter, and take the continuous filtrate as the test solution . Take an appropriate amount of blank exci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com