Preparation method of nevirapine intermediate
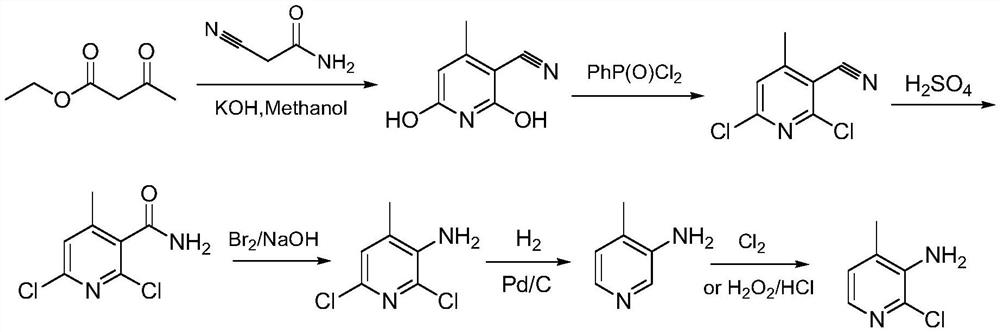
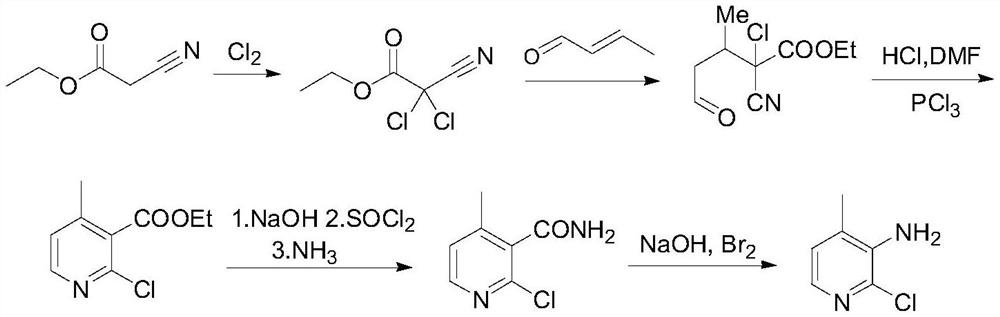
A picoline and amino technology is applied in the preparation of nevirapine intermediate 2-chloro-3-amino-4-picoline, and in the field of nevirapine intermediate preparation, and can solve the problems of long reaction route, low yield and the like, Achieve the effect of low Pd loading, high raw material conversion rate and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 0.5%Pd-1.5%Ru-25%Ni / SiO 2 Catalyst, the preparation method is as follows:
[0038] Step (1), SiO 2 Pretreatment: SiO 2 Roasted at 500℃ for 6h, cooled to room temperature, ground, and sieved to obtain SiO with a size of 100-150 mesh 2 ,spare;
[0039] Step (2), add 100g deionized water and 10g pretreated SiO to the reactor 2 , stirred, heated to 80 °C, took 50 mL of Pd 2+ PdCl at a concentration of 1g / L 2 Aqueous solution, 100mL Ru 3+ RuCl with a concentration of 1.5g / L 3 Aqueous solution, 100mL Ni 2+ Ni(NO) with a concentration of 25g / L 3 ) 2 ·6H 2O aqueous solution, add the three solutions dropwise at the same time and control the drop rate respectively, complete the dropwise addition within 2 hours at the same time, keep the temperature at 60 °C, continue stirring for 4 hours, then add dropwise NaOH solution to adjust the pH to 8-9, continue stirring for 4 hours, filter, The filter cake was washed with deionized water until the filtrate was neutral, dried ...
Embodiment 2
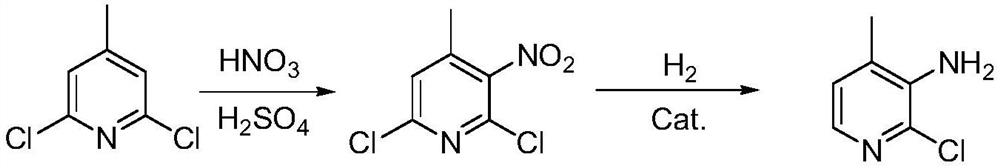
[0041] Under ice bath condition (0~5 ℃), add 2,6-dichloro-4-methylpyridine 16.5g (purity 98.0%, 0.1mol) and concentrated sulfuric acid (mass fraction 98%) 66g into the four-necked flask, Stir for 30min, then slowly add concentrated nitric acid 11.8g (mass fraction 80%, 0.15mol) dropwise, control the drop rate, maintain the temperature not more than 10 ℃, after the dropwise addition, continue to maintain the temperature not more than 10 ℃, stir for 30min, heat up to 60 ℃, the reaction is kept warm and the reaction is controlled in the middle until the reaction of the raw materials is completed. The reaction solution was lowered to normal temperature, slowly added to 150 g of ice water, fully stirred, filtered, the filter cake was washed with water, and dried in vacuum at 40 °C to obtain 2,6-dichloro-3-nitro-4-methylpyridine 20.2 g (purity 95.1%), yield 93.0%.
[0042] 20.2 g of 2,6-dichloro-3-nitro-4-methylpyridine (purity 95.1%, 0.093 mol), 100 g of absolute ethanol and 10.2 ...
Embodiment 3
[0044] 20.2 g of 2,6-dichloro-3-nitro-4-methylpyridine (purity 95.1%, 0.093 mol), 100 g of tetrahydrofuran, and 8.0 g of pyridine (purity 99%, 0.1 mol) were mixed to prepare 2,6- Dichloro-3-nitro-4-methylpyridine was dissolved in tetrahydrofuran, placed in a 250mL autoclave, and 2g of the catalyst (0.5%Pd-1.5%Ru-25%Ni / SiO) prepared in Example 1 was added 2 ), filled with nitrogen and replaced three times, charged to 3MPa, heated to 80°C for the reaction, continuously added hydrogen during the reaction, maintained the hydrogen pressure at 2.5-3.2MPa, kept the reaction at 80°C for 10h, and the reaction was completed; cooled down, released the pressure, filtered out Catalyst, filtrate sampling, HPLC quantitative analysis, the conversion rate of 2,6-dichloro-3-nitro-4-methylpyridine was calculated to be 94.4%, the selection of 2-chloro-3-amino-4-methylpyridine Sex is 90.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com