ATRA-PBAE prodrug copolymer as well as preparation method and application thereof
A technology of copolymers and prodrugs, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of short biological half-life, low bioavailability of ATRA, poor water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
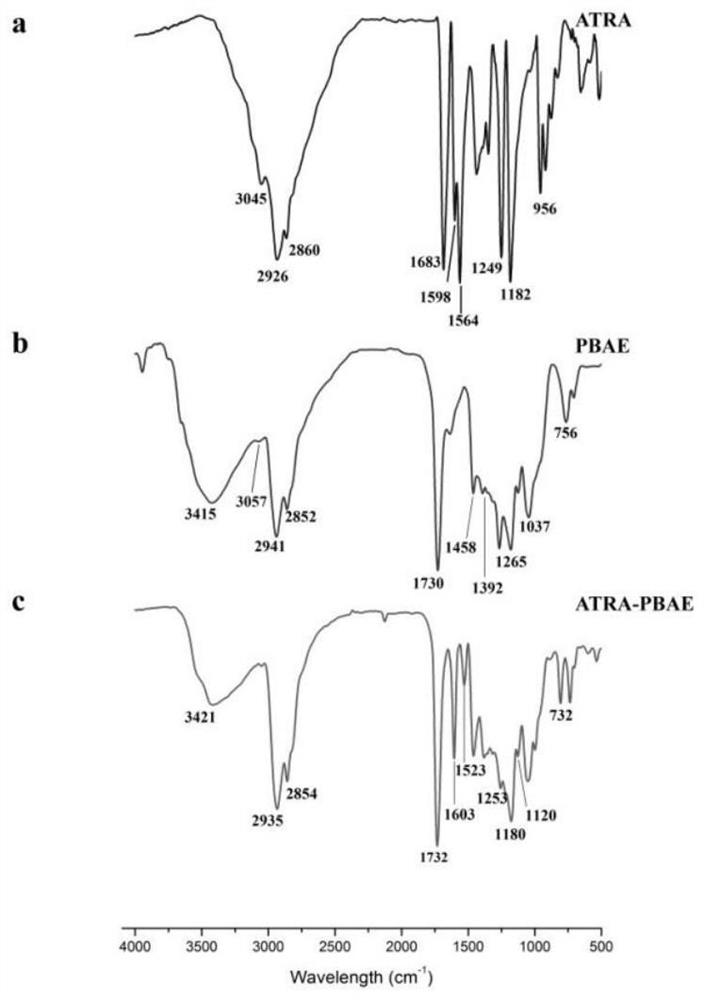
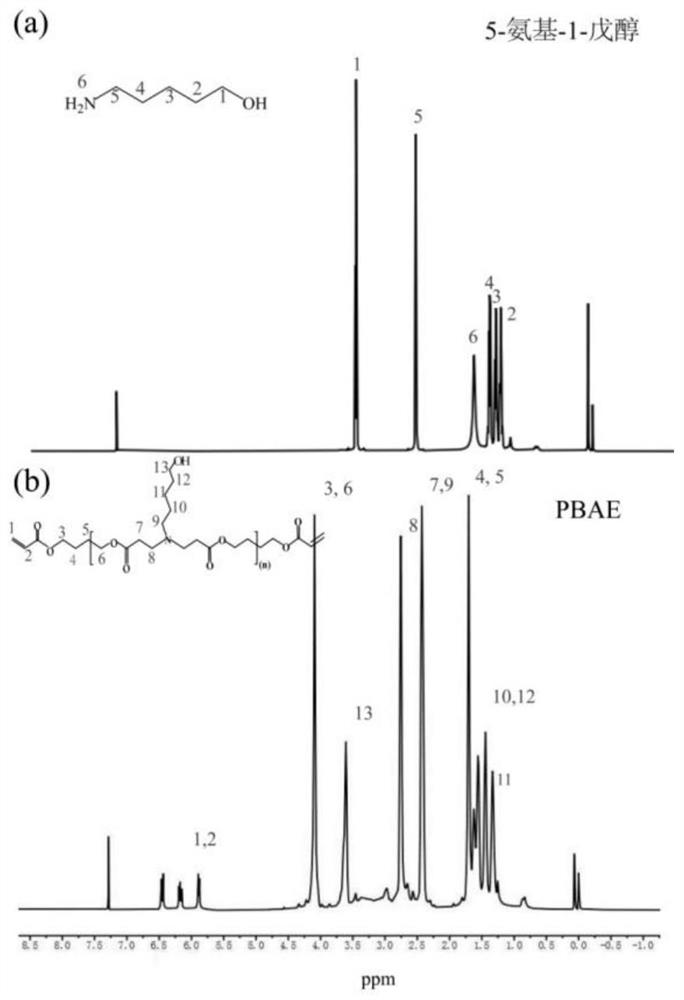
[0047] The present embodiment provides the synthesis and characterization of ATRA-PBAE, and the specific operations are as follows:
[0048] 1. Instruments and Reagents
[0049] The experimental instruments, reagents and materials used in this example are all commercially available products.
[0050] 2. Experimental method
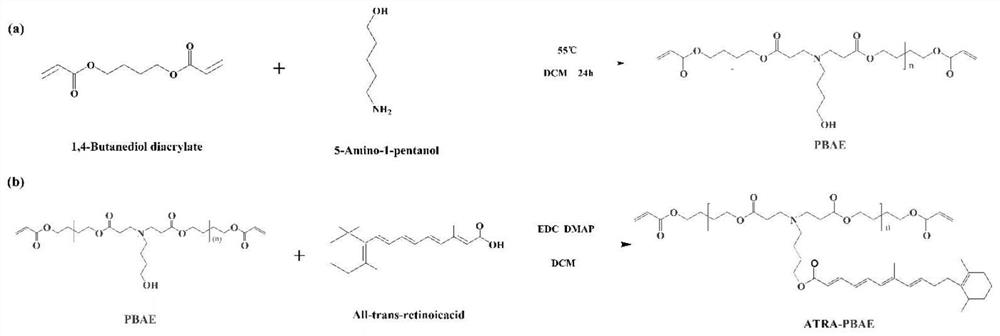
[0051] 2.1 Synthesis of poly(β-aminoester)
[0052] PBAE synthesis is by Michael addition reaction. Under the molar ratio of 1.2:1, 400 mg of 5-amino-1-pentanol and 640 mg of 1,4-butanediol diacrylate were precisely weighed, dissolved in dichloromethane, and stirred magnetically at 55 °C for 48 h. After the reaction, the reaction product was purified according to the following process: the product was placed in a 10 mL EP tube, 5 mL of ether was added, vortexed for 5 min, centrifuged (5600 rpm, 5 min), discarded the upper layer solution, and repeated 3 times. The organic solvent was removed by vacuum drying, and the product was obtained and stored at 4...
Embodiment 2
[0074] In this example, on the basis of the successful synthesis of the ATRA-PBAE prodrug polymer in Example 1, the formulation evaluation of the ATRA-PBAE prodrug nanoparticles was carried out.
[0075] 1. Instruments and materials
[0076] The experimental instruments, reagents and materials used in this example are all commercially available products.
[0077] 2. Experimental part
[0078] 2.1 Selection of HPLC conditions
[0079] Chromatographic column: DiamonsilC18 (4.6×250mm, 5μm); Mobile phase: methanol-water-glacial acetic acid (90:9.5:0.5);
[0080] Column temperature: 25°C; flow rate: 0.8 mL / min; detection wavelength: 340 nm; injection volume: 10 μL.
[0081] 2.2 Preparation of ATRA-PBAE prodrug nanoparticles
[0082] ATRA-PBAE prodrug nanoparticles were prepared by precipitation method. Accurately weigh 10 mg of ATRA-PBAE polymer and dissolve it with 1 mL of acetone; and under the action of magnetic stirring, slowly drop the prodrug acetone solution into an aqu...
Embodiment 3
[0115] This example is the study of the effect of ATRA-PBAE prodrug nanoparticles on tumor heterogeneous cells
[0116] CSCs are a class of tumor heterogeneous cells with high self-renewal ability and differentiation potential. They are closely related to tumorigenesis, recurrence and metastasis, and drug resistance, and are the main reason for breast tumor recurrence. Pin1 is involved in multiple mechanistic pathways of breast cancer tumors, and Pin1 is not only overexpressed in breast cancer cells, but also a key regulator of BCSCs. Therefore, regulating the activity of Pin1 can effectively control the growth or inhibition of breast tumor cells.
[0117] In this example, based on the successful preparation of ATRA-PBAE nanoparticles, the proliferation inhibitory effect and cellular uptake of ATRA prodrug nanoparticles on MCF-7 cells and MS cells were further studied, and the two cellular uptake mechanisms and drug effects in cells were investigated. transfer within.
[011...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com