Medicinal composition for treating cardiovascular and cerebrovascular diseases and preparation method thereof
A technology for cardiovascular and cerebrovascular diseases and a composition, which is applied in the field of pharmaceutical preparations, can solve the problems of decreased absorption, poor water solubility of scutellarin, and poor bioavailability of scutellarin, and achieves high bioavailability, simple preparation method, good absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
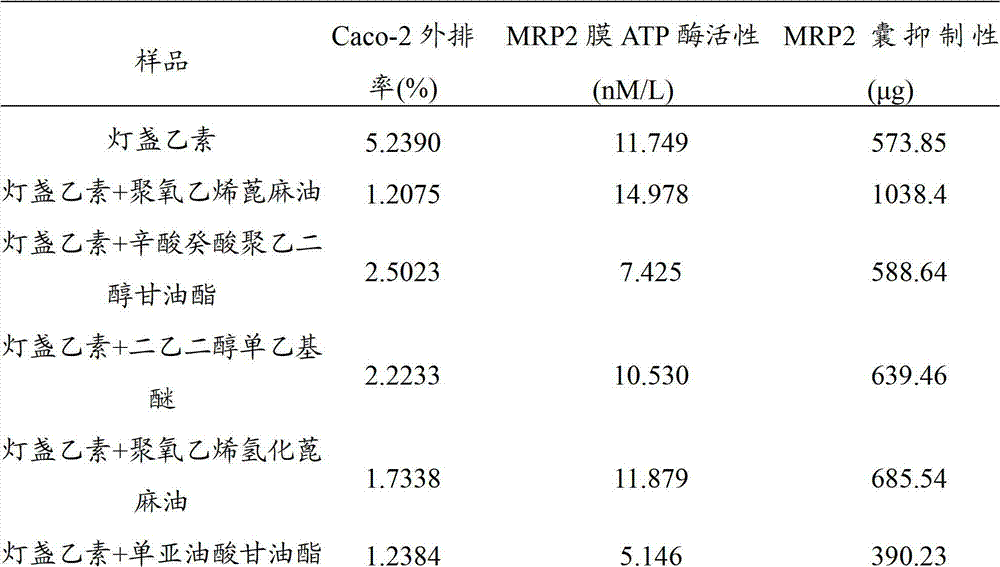
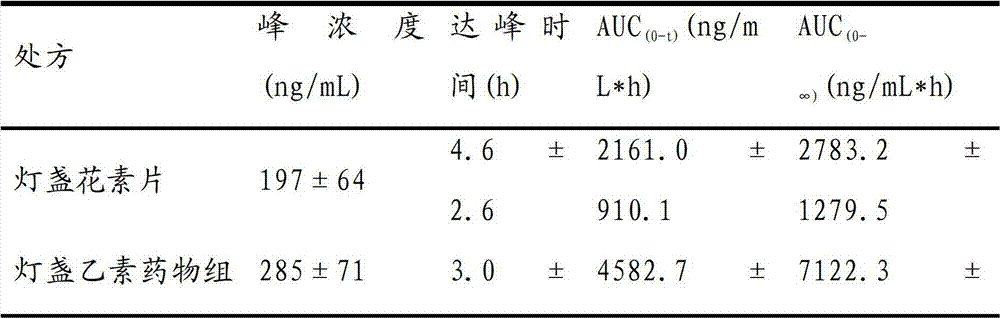
[0041] A pharmaceutical composition for treating cardiovascular and cerebrovascular diseases is prepared from the following components in percentage by weight: co-emulsifier 24%, emulsifier 47.7%, oil phase 28% and drug 0.3%.
[0042] The drug is scutellarin.
[0043] Described co-emulsifier is diethylene glycol monoethyl ether.
[0044] The emulsifier is a mixture of polyoxyethylene castor oil and caprylic capric macrogol glyceride (1:1 by weight).
[0045] The oil phase is a mixture (weight ratio: 1:1) of monolinoleic glyceride and coconut oil caprylic capric glyceride (Capmul MCM, purchased from Abitec, USA, the same below).
[0046] Preparation:
[0047] First, mix the co-emulsifier and emulsifier of diethylene glycol monoethyl ether, polyoxyethylene castor oil and caprylic capric acid macrogol glyceride evenly, then disperse scutellarin in it, and then slowly add Mix the oil phase of monolinoleic acid glyceride and coconut oil caprylic capric acid glyceride (Capmul MCM...
Embodiment 2
[0049] A pharmaceutical composition for treating cardiovascular and cerebrovascular diseases is prepared from the following components in weight percent: co-emulsifier 23.6%, emulsifier 46.3%, oil phase 30% and drug 0.1%.
[0050] The drug is scutellarin.
[0051] Described co-emulsifier is diethylene glycol monoethyl ether.
[0052] The emulsifier is a mixture of polyoxyethylene hydrogenated castor oil and caprylic capric macrogol glyceride (4:1 by weight).
[0053] The oil phase is a mixture of monolinoleic acid glyceride and coconut oil caprylic capric acid glyceride (Capmul MCM) (weight ratio is 2:1).
[0054] Preparation:
[0055] First disperse scutellarin in the oil phase of glyceryl monolinoleate and coconut oil caprylic capric acid glycerin (Capmul MCM) mixed uniformly, and then slowly add diethylene glycol monoethyl ether, polyoxyethylene The co-emulsifier and emulsifier of hydrogenated castor oil and caprylic capric acid macrogol glyceride are stirred at a consta...
Embodiment 3
[0057] A pharmaceutical composition for treating cardiovascular and cerebrovascular diseases is prepared from the following components in weight percentage: co-emulsifier 20%, emulsifier 49.9%, oil phase 30% and drug 0.1%.
[0058] The drug is scutellarin.
[0059] Described co-emulsifier is diethylene glycol monoethyl ether.
[0060] The emulsifier is a mixture of polyoxyethylene castor oil, polyoxyethylene hydrogenated castor oil and caprylic capric macrogol glyceride (weight ratio is 1:1:1).
[0061] The oil phase is glycerol monolinoleate.
[0062] Preparation:
[0063] First disperse scutellarin in glycerol monolinoleate, then slowly add diethylene glycol monoethyl ether, polyoxyethylene castor oil, polyoxyethylene hydrogenated castor oil and caprylic capric acid macrogol glycerin Co-emulsifier and emulsifier of ester, constant temperature magnetic stirring at 37°C to mix the components evenly, completely dissolve the drug, and obtain the scutellarin pharmaceutical com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com