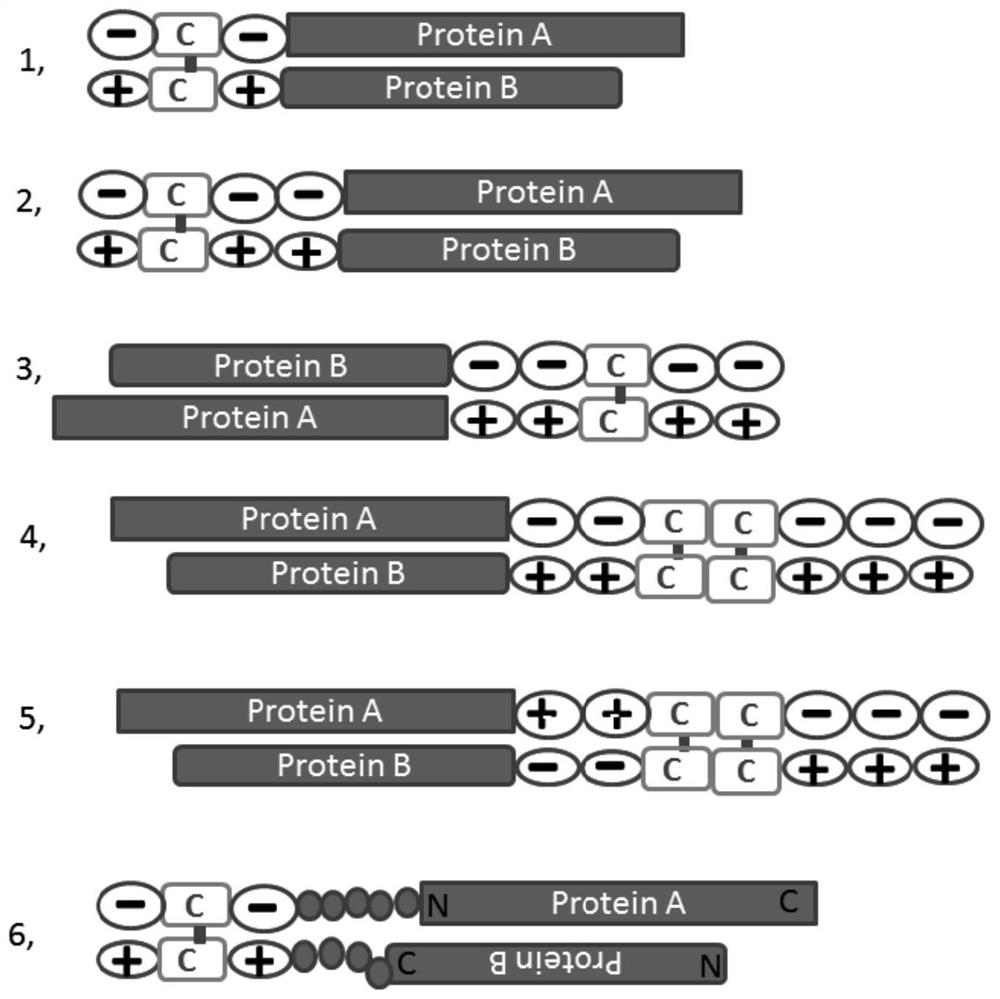
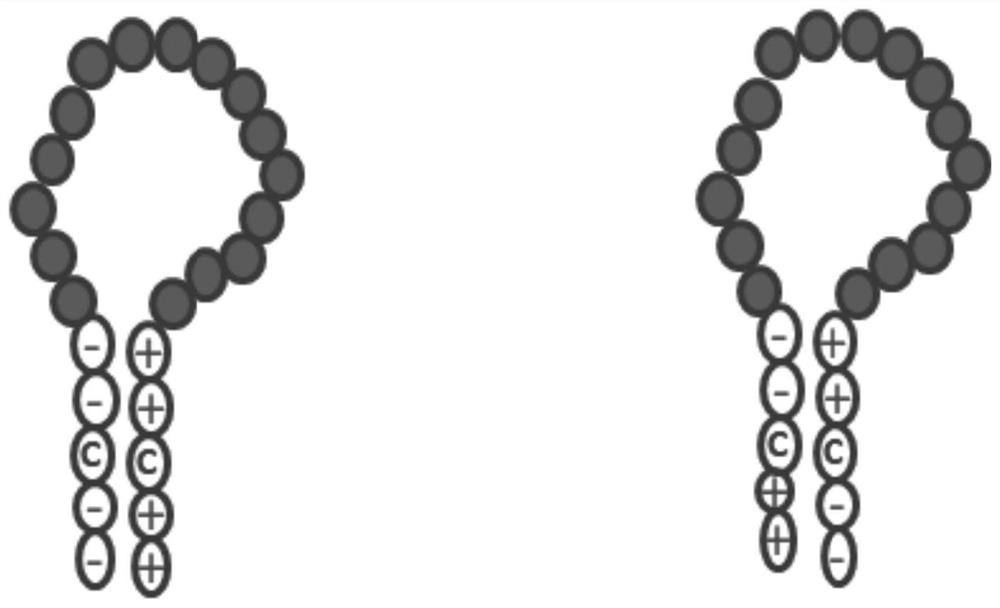
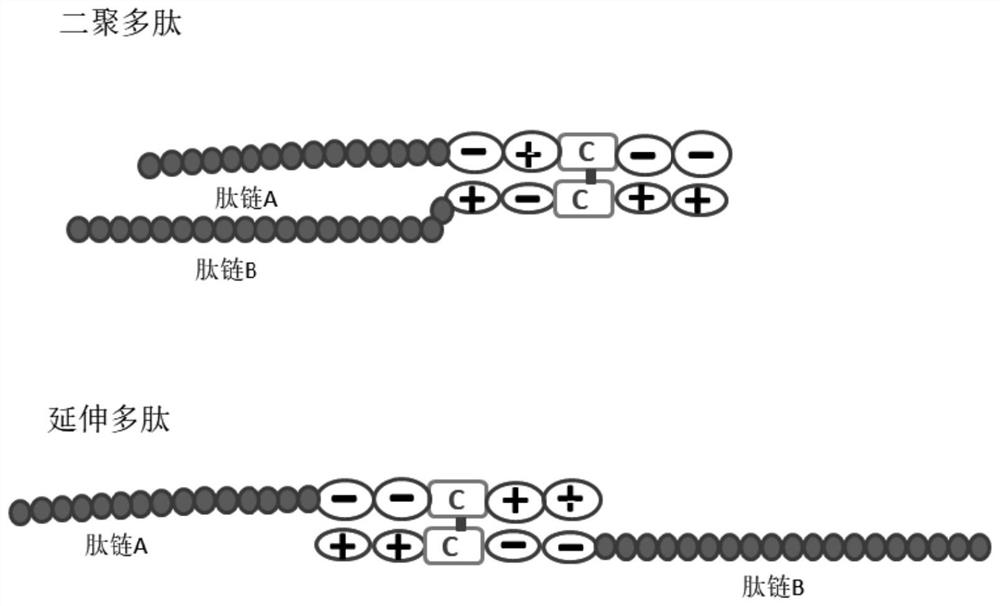
Zipper fastener structure for promoting formation of protein dimer and application of zipper fastener structure
A technology of dimers and zipper buttons, which is applied in the detection of general structure details of gas analyzers, expression enhancement stability/folded protein fusion, antibody mimics/stents, etc., can solve problems such as excessive benefits and inappropriateness, and achieve The effect of reducing the chance of multimer formation, improving solubility, and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Construction of expression vector of zipper-type dimer protein ESAT6-CFP10
[0104] A negatively charged amino acid group is added to the C-terminus of ESAT6 and a positively charged amino acid group is added to the C-terminus of the expression gene of CFP10, and a histidine purification tag is added to the front end of the CFP10, wherein:
[0105] The amino acid sequence of ESAT6 after expression is: DYKDDDDKGG (SEQ ID NO.: 3)-MAEMKTDAATLAQEAGNFERISGDLKTQIDQVESTAGSLQGQWRGAAGTAAQAAVVRFQEAANKQKQELDEISTNIRQAGVQYSRADEEQQQALSSQMGF-GGDDCDD (SEQ ID NO.: 1);
[0106] The amino acid sequence of CFP10 after expression is: HHHHHHGG (SEQ ID NO.: 4) - MTEQQWNFAGIEAAASAIQGNVTSIHSLLDEGKQSLTKLAAAWGGSGSEAYQGVQQKWDATATELNNALQNLARTISEAGQAMASTEGNVTGMFA-GGKKCKK (SEQ ID NO.: 2);
[0107] The sequence-added ESAT6 and CFP10 gene fragments were synthesized and inserted into the two ends of the IRES sequence in the pET expression vector to form an expression plasmid. The purified expressio...
Embodiment 2
[0144] Example 2: Application of Cyclic Peptide Design in CCP Detection:
[0145] On the basis of the traditional CCP, the position of the disulfide bond was changed, and the dimer zipper was added on both sides of the polypeptide, and the new polypeptide structure was KKCK-CCP-DCDD; The detection rate of autoimmune antibodies in China has increased by 10%. It shows that the stability of cyclic formation is very important for the detection sensitivity of CCP, and increasing the stability of the cyclic peptide can further improve the sensitivity of CCP in the detection of citrullinated autoantibodies.
[0146] The samples were coated with streptavidin-dimer zipper loop peptide CCP at a concentration of 5µg / ml, and the samples were detected after blocking with non-fat milk powder. The secondary antibody was HRP-goat anti-human. The control reagent was the European Diagnostics CCP ELISA test kit.
[0147] like Figure 10 As shown, among 95 RA patients, the detection rate of CC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com