Primer and probe combination for simultaneously detecting three infectious bovine pathogens
A probe and primer sequence technology, which is applied in the application field of the primer and probe combination, can solve the problems of lengthy operation, cumbersome operation, and long time, so as to improve accuracy and sensitivity, broad application space, and improve detection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The design of the primer of embodiment 1
[0036] All nucleotide sequences of BVDV-5'UTR; BRV-VP6; BCV-N gene region were downloaded from GenBank, and the MEGA software was used for alignment analysis to select the most conserved gene sequence (BVDV GenBank accession number: LC099927.1 ; BRV GenBank accession number: AB374146.1; BCV GenBank accession number: LC494138.1) and its specific region, designed and screened specific primers and probes (Table 1).
[0037] Table 1 Primers and probes
[0038]
Embodiment 2
[0039] Example 2 Establishment and optimization of multiplex real-time fluorescent quantitative PCR method
[0040] 1. Establishment and optimization of single detection real-time quantitative PCR method for BVDV, BRV and BCV pathogens
[0041] 1.1 Standard positive plasmid preparation
[0042] Using the primers in Table 1, BVDV-5'UTR; BRV-VP6; BCV-N gene region (BVDV GenBank Accession No.: LC099927.1; BRV GenBank Accession No.: AB374146.1; BCV GenBank Accession No.: LC494138.1) A segment of the sequence including the primer sequence was inserted into the pUC57 vector to form a recombinant plasmid, named pUC57-5'UTR, pUC57-VP6, pUC57-N as the standard positive plasmid template and positive control in the system construction.
[0043] 1.2 Establishment of single-detection real-time fluorescent quantitative PCR method
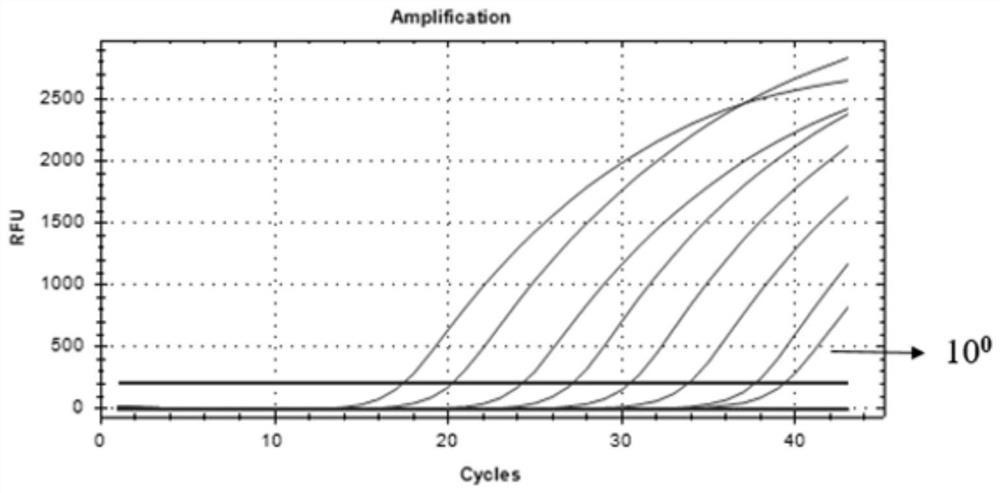
[0044] A single factor variable was set, and according to the primers and probes in Table 1, real-time quantitative PCR was used to optimize the primer concent...
Embodiment 3 3
[0094] Example 3 Evaluation of the triple real-time fluorescence quantitative PCR system for the detection of clinical samples
[0095] Using the triple real-time fluorescence quantitative PCR system established in this study and the common PCR detection method obtained from the literature review (Table 10), 143 cDNA samples stored in the laboratory were tested for clinical samples and the coincidence rate was analyzed. The results of each common PCR test were verified by sequencing of Wuhan Qingke Biotechnology Co., Ltd.; the criteria for the triple real-time PCR detection method were:
[0096] 1. The Ct value of the tested sample is less than 40 and it is judged to be positive when a typical amplification curve appears;
[0097] 2. When the Ct value of the tested sample is greater than 40 and there is a typical amplification curve, it needs to be re-tested. If the re-examination result is as above, it will be judged as positive, otherwise it will be judged as negative;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com