Oncolytic virus therapy with induced anti-tumor immunity
A technology of anti-tumor immunity and oncolytic virus, which is applied in the field of viral tumor therapy and can solve the problems of limited oncolytic activity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Construction of the exposed instance
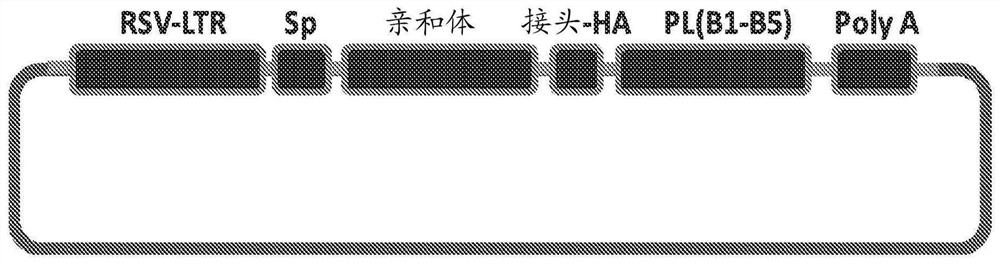
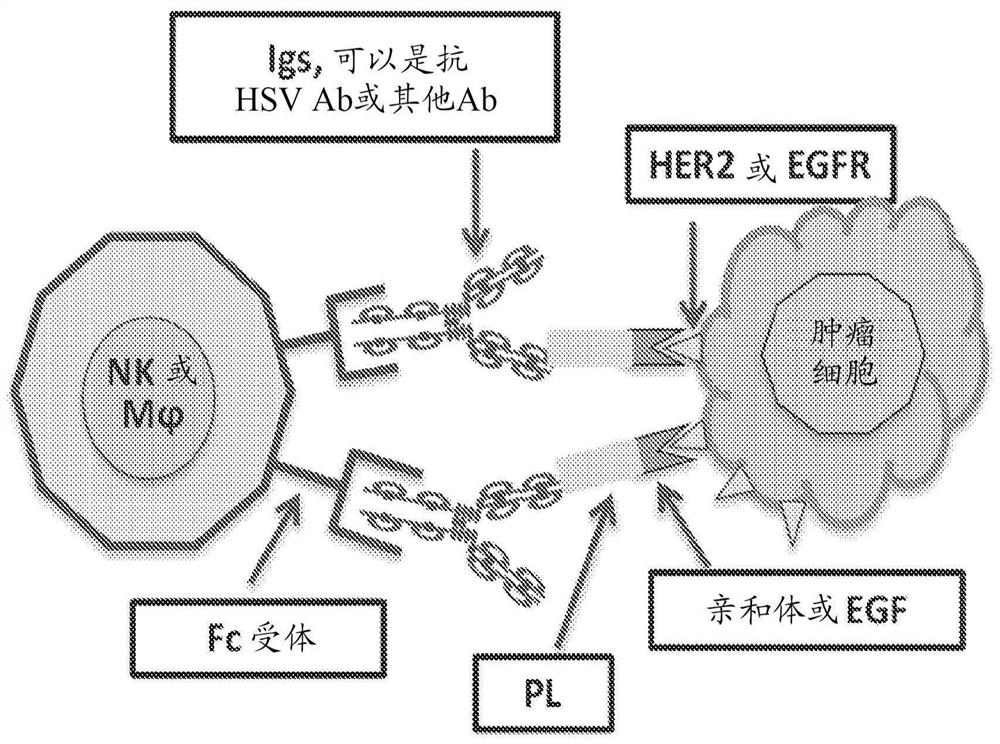
[0066] Figure 1A and Figure 1B The design of exemplary affibosome-PLs and their in vivo mechanisms of action in the tumor microenvironment are described. Affibody molecules are short peptides of 58 amino acids in length and are based on triple alpha-helical Z domain scaffolds that can be selected from combinatorial libraries to bind specific protein targets with strong affinity and specificity. See Feldwisch J, et al. "Engineering of affibody molecules for therapy and diagnostics" Methods Mol Biol. 899 (2012) 103-26. Examples of suitable affibodies selected for strong binding affinity to HER2 are available. See Orlova A, et al. "Tumor imaging using a picomolar affinity HER2 binding affibody molecule" Cancer Res. 66(8)(2006) 4339-48; Steffen AC, et al. "Affibody-mediated tumor targeting of HER-2 expressing xenografts in mice" Eur J Nucl Med Mol Imaging 33(6) (2006) 631-8. The coding sequences from the five immunoglobulin bindi...
Embodiment 2
[0072] Affibody-PL engagement of innate immune cells
[0073] Efforts were made to demonstrate that Affibody-PL can actively engage innate immune cells to attack tumor cells when tested in vitro. First, the ability of Affibody-PL to selectively bind to HER2-expressing tumor cells was examined. The plasmid or control plasmid (pcDNA3-EGFP from Addgene) containing the gene cassette pcDNA-Affibody-PL constructed by inserting Affibody-PL into the pcDNA3 plasmid was transfected into 293 cells for 24 hours (hr) Then collect the supernatant. Supernatants (100 μl) were added to three tumor cell lines (Skov3, from serous cystadenocarcinoma; MCF7; and MDA-MB-231) expressing varying levels of HER2 to allow Affibody-PL to bind tumors first HER2 on the cell surface. After washing, FITC-conjugated anti-HA-tagged antibody was added. The stained cells were then analyzed by flow cytometry and the results were shown in image 3 middle. As shown, Affibody-PL efficiently bound Skov3 cells ex...
Embodiment 3
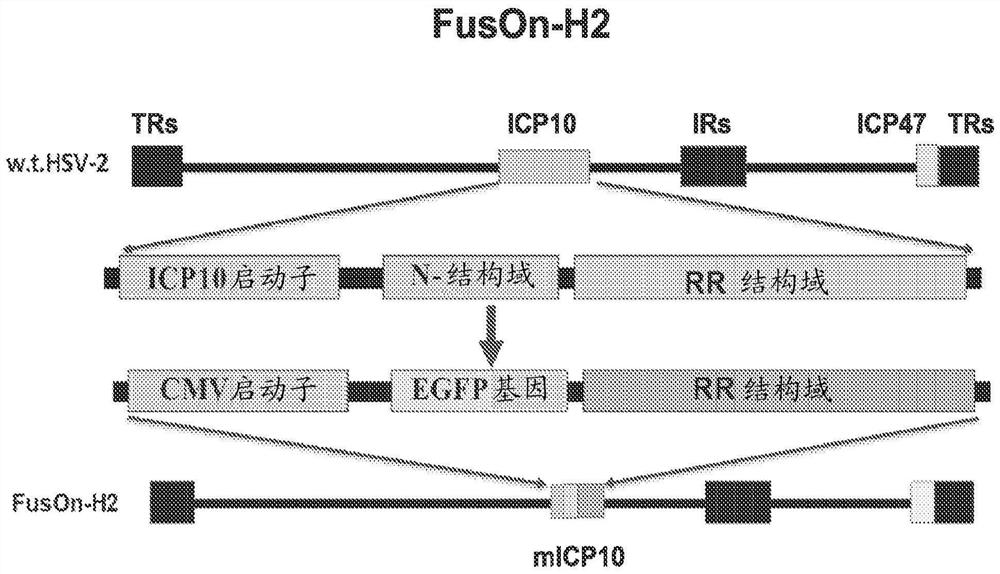
[0076] Insertion of Affibody-PL coding sequences into oncolytic HSV and in vitro identification of the equipped virus.
[0077] Next, by techniques previously described in certain inventors' publications (see Fu, X et al.), by methods such as Figure 2A and Figure 2B Homologous recombination shown inserts the Affibody-PL coding sequence into the genome of the HSV-2 based oncolytic virus FusOn-H2. FusOn-H2 is a herpes simplex virus type 2 mutant that lacks the protein kinase domain of the ICP10 gene and is a potent oncolytic virus (Mol Ther. 13(5) (2006) 882-90; Fu X, et al. “Construction of anoncolytic herpes simplex virus that precisely targets hepatocellular carcinoma cells" Mol Ther. 20(2)(2012) 339-46); each of which is incorporated herein by reference. The complete sequence of the derived FusOn-Affibody-PL is provided in SEQ ID NO:17. Expression of Affibody-PL from the novel virus FusOn-PL was confirmed by Western blot analysis of supernatants collected from virus-inf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com