Method for preventing human glands from radiation injury
A technology for radiation damage and glands, applied in the field of radiation oncology to prevent side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
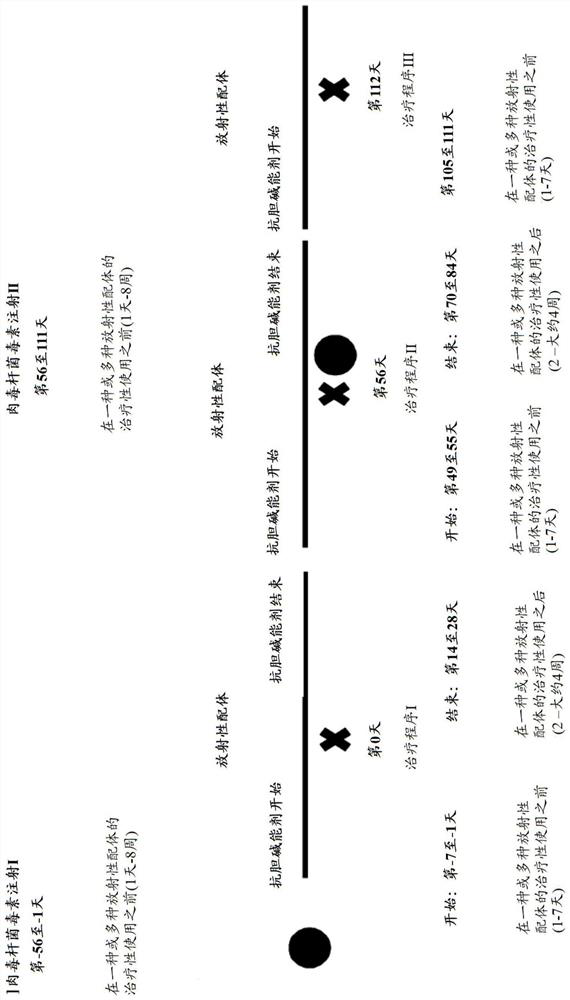
[0072] The methods of the present invention are suitable for a variety of applications. It is not only suitable for diagnosing diseases, for which radionuclides or radioligands are used; but also for the treatment of diseases, for which radionuclides or radioligands are used. Exemplary radionuclides include 32 P. 60 Co. 90 Sr, 90 Y. 103 Pd, 125 I. 131 I. 137 Cs, 188 Re, 192 Ir, 198 Au, 226 Ra. Diseases include neoplastic diseases such as bronchial cancer (eg small cell or large cell bronchial carcinoma), colon cancer, breast cancer, prostate cancer, liver cancer, pancreatic cancer, bladder cancer, skin cancer, ovarian cancer, genitourinary tract cancer, adrenal cortex cancer (pheochromocytoma), brain cancer, stomach cancer, kidney cancer, uterine cancer, bone cancer, esophagus cancer, oropharyngeal cancer, testicular cancer, thyroid cancer, adrenocortical cancer, gallbladder cancer, small bowel cancer, anal cancer, pancreatic cancer, Cholangiocarcinoma, cervical c...
Embodiment 1
[0101] Example 1: Use 68 Example of Ga-PSMA, by use of botulinum toxin type A in the diagnostic use of radioligands Bacteriotoxin and anticholinergic drugs to prevent radiation injury
[0102] in an imaging procedure 68 Two weeks prior to diagnostic use of Ga-PSMA, depending on the specific botulinum toxin type A preparation, a total of between 20 and 900 units of botulinum toxin type A, when used or For example 150 units are applied to the relevant glands, such as one or both mandibular glands and / or one or both parotid glands. In use 68 Three days prior to the imaging procedure for Ga-PSMA, additional administration of anticholinergic drugs was initiated. The anticholinergic drug (scopolamine in this case) is administered in the form of one to two transdermal patches every three days. In this case, about 1 mg of scopolamine is released into the systemic circulation at an almost constant rate over 72 hours. Alternatively, the anticholinergic drug is administered o...
Embodiment 2
[0103] Example 2: Use 68 Example of Ga-PSMA, by use of botulinum toxin type B in the diagnostic use of radioligands Bacteriotoxin and anticholinergic drugs to prevent radiation injury
[0104] in an imaging procedure 68 Two weeks prior to diagnostic use of Ga-PSMA, a total of 3,000 units of botulinum toxin type B (eg ) to the desired glands, such as the submandibular glands on one or both sides, and / or in the parotid glands on one or both sides. In use 68 Three days prior to the imaging procedure for Ga-PSMA, additional administration of anticholinergic drugs was initiated. The anticholinergic drug, here scopolamine, is applied in the form of one to two transdermal patches every three days. In this case, about 1 mg of scopolamine is released into the systemic circulation at an almost constant rate over 72 hours. Alternatively, the anticholinergic drug is administered orally daily (two days before the diagnostic procedure, one day before the diagnostic procedure, on t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com