Method for measuring contents of zinc, cadmium, tin and copper in silver-based brazing filler metal
A technology for silver-based solder and copper content, applied in the field of chemical analysis, can solve the problems of inconsistent sample processing methods, complicated copper separation process, experimental errors, etc., to meet the needs of fast and accurate detection, the determination method is fast and simple, and the analysis is shortened. effect of cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
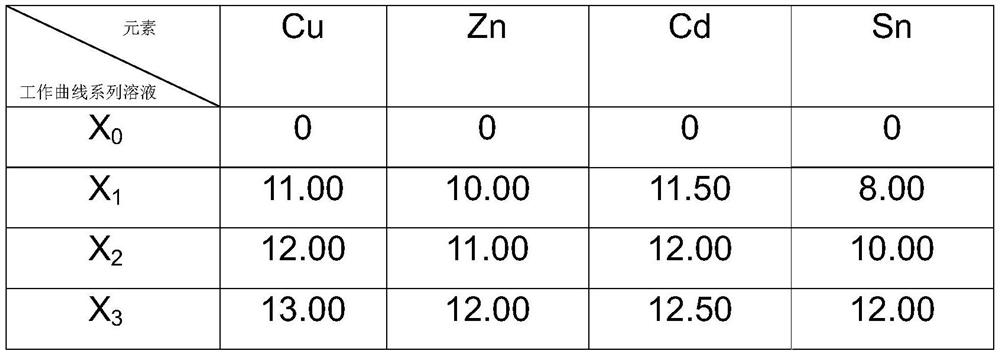
[0042] This embodiment is a method for jointly measuring the contents of Zn, Cd, Cu and Sn in BAg40CuZnCdSn.
[0043] Content range: Zn: 10.0% to 11.5%, Cd: 23.0% to 25.0%, Cu: 23.5% to 25.5%, Sn: 0.8% to 1.2%
[0044] Weighing of BAg40CuZnCdSn samples. Weigh 0.1017g sample and place it in a 250mL polytetrafluoroethylene beaker. Accurately weighed samples of BAg40CuZnCdSn were obtained.
[0045] Dissolution of BAg40CuZnCdSn sample. Add 10 mL of nitric acid to the 250 mL polytetrafluoroethylene beaker of the accurately weighed sample, put the beaker on an electric furnace and heat it up to 50°C for heating and dissolving. During the dissolving process, add 3 drops of hydrofluoric acid dropwise. After the solution is clear, add 10mL of hydrochloric acid and 50mL of distilled water were heated and boiled on an electric stove, removed after the silver chloride was coagulated, and cooled to room temperature naturally. The dissolved BAg40CuZnCdSn sample solution was obtained.
...
Embodiment 2
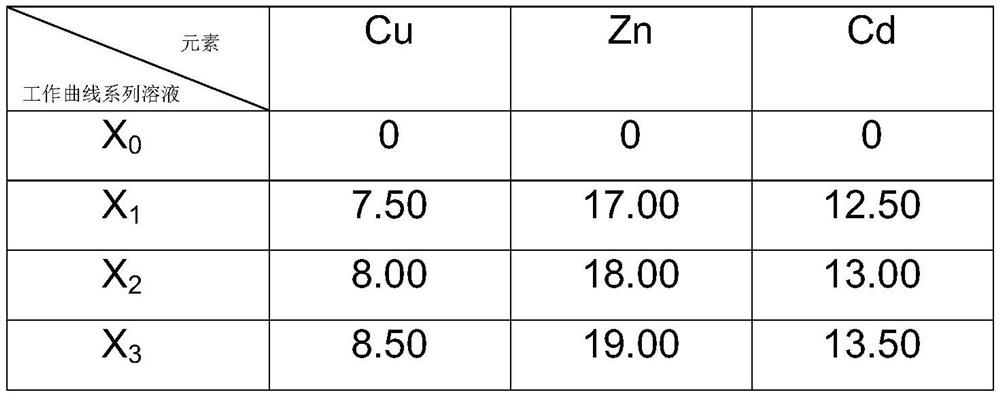
[0058] This embodiment is a method for jointly measuring the contents of Zn, Cd and Cu in BAg40CdZnCu(Ni)-S.
[0059] Content range: Zn: 17.3% to 18.8%, Cd: 25.1% to 26.5%, Cu: 15.5% to 16.5%
[0060] Weighing of BAg40CdZnCu(Ni)-S alloy samples. Weigh 0.1024g sample and place it in a 250mL polytetrafluoroethylene beaker. Accurately weighed samples of BAG40CDZNCU(NI)-S alloy were obtained.
[0061] Dissolution of BAg40CdZnCu(Ni)-S alloy samples. Add 10 mL of nitric acid to the 250 mL polytetrafluoroethylene beaker of the accurately weighed sample, put the beaker on an electric furnace and heat it up to 50 °C to heat and dissolve, when the solution is clear, add 10 mL of hydrochloric acid and 50 mL of distilled water, and heat and boil on the electric furnace , remove the silver chloride after condensation, and cool to room temperature naturally. The dissolved BAg40CdZnCu(Ni)-S alloy sample solution was obtained.
[0062] Constant volume of BAg40CdZnCu(Ni)-S alloy sample so...
Embodiment 3
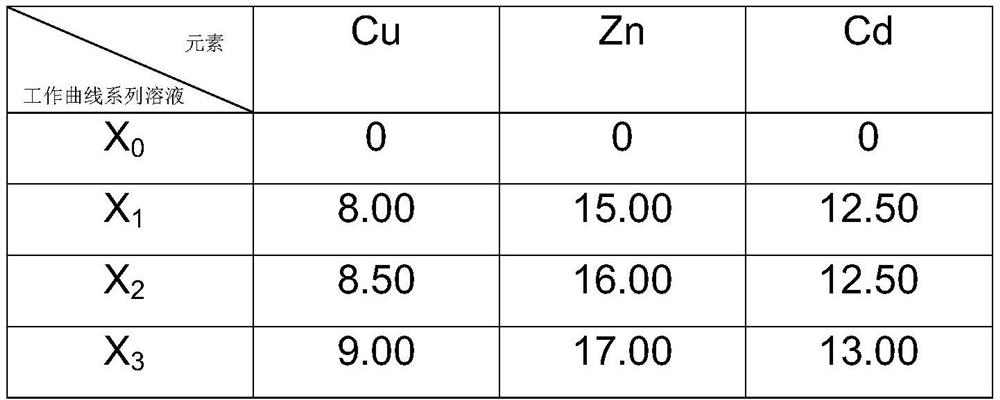
[0074]This embodiment is a method for jointly measuring the contents of Zn, Cd and Cu in HBAg42CdCuZn.
[0075] Content range: Zn: 15.0% to 17.0%, Cd: 24.0% to 26.0%, Cu: 16.0% to 18.0%
[0076] Weighing of HBAg42CdCuZn sample. Weigh 0.1005g sample and place it in a 250mL polytetrafluoroethylene beaker. Accurately weighed samples of HBAg42CdCuZn were obtained.
[0077] Dissolution of HBAg42CdCuZn sample. Add 10 mL of nitric acid to the 250 mL polytetrafluoroethylene beaker of the accurately weighed sample, put the beaker on an electric furnace and heat it up to 50 °C to heat and dissolve, when the solution is clear, add 10 mL of hydrochloric acid and 50 mL of distilled water, and heat and boil on the electric furnace , remove the silver chloride after condensation, and cool to room temperature naturally. The dissolved HBAg42CdCuZn sample solution was obtained.
[0078] The constant volume of the HBAg42CdCuZn sample solution. Rinse the bottle wall of the dissolved sample ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com