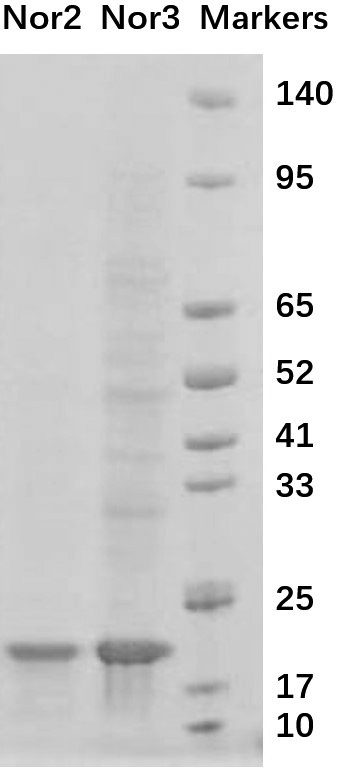
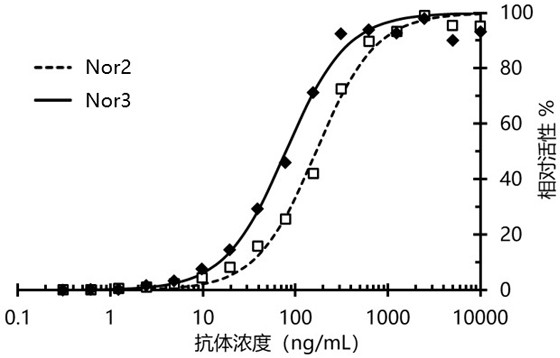
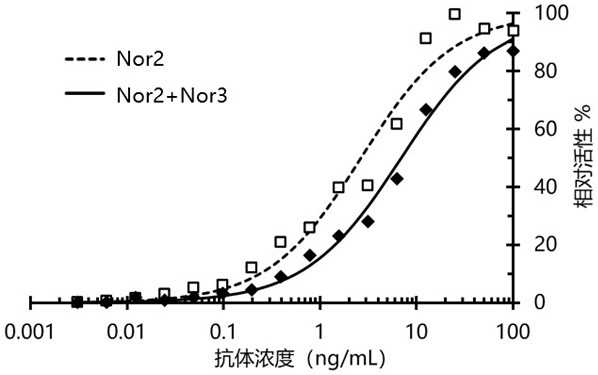
Nanometer antibody targeting norovirus protein and application thereof
A nanobody and protein technology, applied in the field of antibody technology and biomedicine, can solve the problems of high immunogenicity, long production cycle, vacancy of nanobody, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Panning of phage display library
[0022] According to the method of binding, washing, elution and amplification, the phage display library was subjected to affinity panning, and the Norovirus capsid protein VP1 antigen was used as the antigen for panning targeting Norovirus Nanobodies, and the Alternate blocking with no blocking and 5% milk to reduce nonspecific adsorption. The specific steps of panning are as follows. Add 100 μL of viral protein antigen at a concentration of 10 μg / mL (1 μg / well) to the wells of the eight-continuous-well enzyme-labeled strip, and coat at 37 °C for 2 hours. The supernatant was discarded, and the enzyme-labeled strip was washed 5 times with 0.05% PBST. For blocking, add 200 μL of 5% milk to each well and block for 2 hours at 37 °C. The blocking solution was discarded, and the ELISA strip was washed 5 times with 0.05% PBST. Add 100 μL of phage library suspension to each well (the volume is 8×10 9 pfu, titer of 1×10 9 cf...
Embodiment 2
[0023] Embodiment 2. Identification of positive clones
[0024] A single colony was randomly picked from the titered plate for phage rescue and purification, and then positive clones were identified by ELISA. Specific steps are as follows. Add 100 μL of viral protein antigen at a concentration of 10 μg / mL (1 μg / well) to the wells of the eight-continuous-well enzyme-labeled strip, and coat at 37 °C for 2 hours. The supernatant was discarded and the ELISA strip was washed 5 times with PBST. Add 200 μL of 5% milk to each well, and make another strip as a negative control, and block at 37 °C for 2 hours. The enzyme-labeled strips were washed 5 times with PBST. Add 100 μL of amplified monoclonal phage suspension to each well, and incubate with shaking at room temperature for 1 hour. Plates were washed 5 times with PBST. Add 100 μL of mouse anti-human influenza hemagglutinin (HA) primary antibody to each well, and incubate with shaking at room temperature for 1 hour. Plates we...
Embodiment 3
[0025] Example 3. Fusion of Nanobodies Nor2 and Nor3 with Histidine Tag and Human Influenza Virus Hemagglutinin Tag
[0026] In the phage display library, the nanobody is fused to the capsid protein P3 of the M13 phage and displayed on the surface of the phage particle. We cut the base sequences of the obtained Nanobodies Nor2 and Nor3 into gene fragments with restriction enzymes NdeI and XhoI, inserted them into the pET23a plasmid, and introduced a histidine tag and human influenza virus blood into the C-terminus of the Nanobodies. The lectin tag facilitates the separation, purification and identification of recombinant proteins using nickel columns. The C-terminal amino acid sequence of the fusion protein of Nanobodies Nor2 and Nor3 is: GQAGQHHHHHHGAYPYDVPDYALEHHHHHH*.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com