Enzymes for cannabinoid synthesis and methods of making and using same
A kind of cannabinoid, cannabinoid phenolic acid technology, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0145] Cloning, Expression and Purification of Prenyltransferases
[0146] In certain embodiments, gene candidates are expressed in E. coli, protein extracts are prepared, and purified enzymes are delivered for testing by affinity enzymatic purification using HIS tags to form CBGAs. In yet other exemplary embodiments, gene candidates are expressed in yeast. Expressed protein extracts were purified and used for testing. Illustrative examples demonstrating specific methods of cloning, expressing and purifying the enzymes of the present disclosure are described in the Examples herein.
[0147] Method for preparing cannabinoids
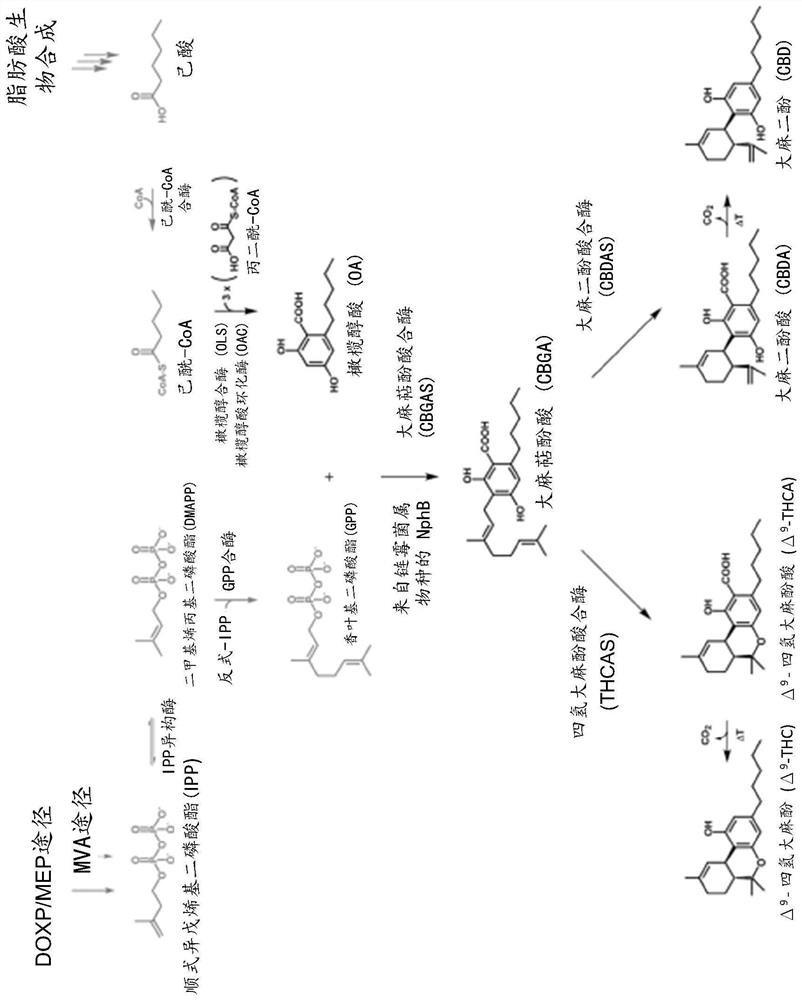
[0148] Cannabinoids can be produced using heterologous expression in microorganisms or yeast. Microorganisms can be genetically engineered to express cannabinoids or cannabinoid precursor molecules. Methods of heterologous expression of cannabinoid compounds are known in the art and are described, for example, in Carvalho et al., 2017, which is incorp...
example 1
[0151] Example 1: Cloning, Expression and Purification of Enzymes
[0152] The candidate gene was purchased from General Biosystems (Anhui, China) Corporation Limited as a gene block, and was located at the restriction endonuclease site NdeI ( CATATG) and XhoI (C TCGAG). All plasmids were transformed into BL21 (DE3) and named eCAN20005 to eCAN20060 (see Table 1).
[0153] 0.5 mL of the saturated culture in LB medium with 50 μg / mL kanamycin was inoculated into 50 mL of TB medium with 50 μg / mL kanamycin. Cultures were grown at 37°C to an OD600 of 0.5-0.8, induced with 0.5 mM IPTG, and then expressed at 25°C for 18 hours at 220 rpm.
[0154] Cells were harvested by centrifugation at 2500 x g, washed with lysis buffer (50 mM Tris-HCl, 500 mM NaCl, [pH 8.0], 10% (v / v) glycerol) and resuspended to OD550 in 10 ml of lysis buffer about 100. Cells were lysed using an ultrasonic cell disruptor for 10 min at 4°C. Lysates were clarified by centrifugation at 12,000 x g for 30 min at...
example 2
[0155] Example 2: In vitro enzyme assay
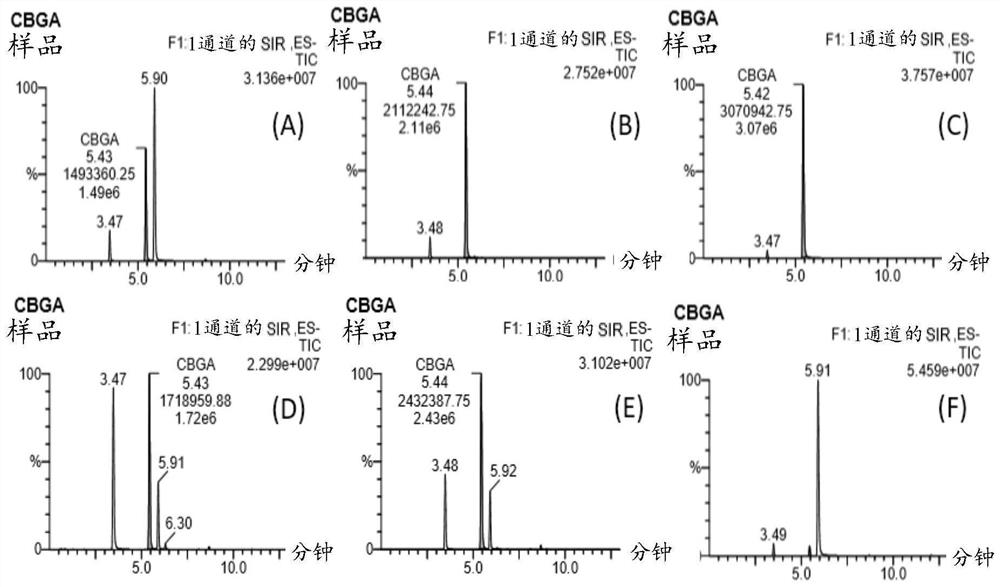
[0156] The reaction conditions for the enzyme assay consisted of the following: 50 mM HEPES (pH=7.5) with 5 mM MgCl2, 2 mM GPP, 2 mM olivetolic acid and 1 mg / ml of purified candidate enzyme in a final volume of 200 uL. After 18 hours of incubation at room temperature, the reaction mixture was extracted twice with 200 μL of ethyl acetate / formic acid (0.05% (v / v)). The organic extracts from each reaction were combined and solvent removed using a block heater. Samples were dissolved in 100 μL of resuspension (acetonitrile / H2O / formic acid (80% / 20% / 0.05% (v / v / v))) and filtered through a 0.22 μm PVDF membrane prior to LC-MS analysis.
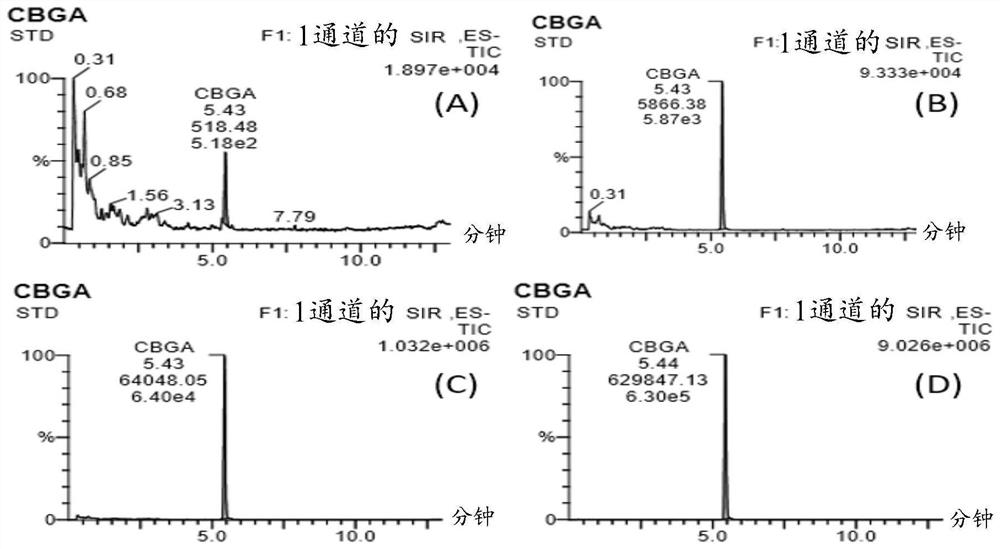
[0157] figure 1 The enzymatic assay was validated by showing the results of titrating CBGA at four different concentrations. CBGA titration curve concentration: (A) 10ng / mL, (B) 100ng / mL, (C) 1ug / mL, (D) 10ug / mL. Table 2 also shows the LC-MS chromatogram of CBGA at m / z 359.5 with a retention time of 5.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com