Method for synthesizing calcitriol through oxidase hydroxylation
A technology of oxidase hydroxylation and calcitriol, which is applied in fermentation and other directions, can solve the problems of not being able to meet the concept of green synthesis, complicated separation and purification, and low yield, and achieve high atom economy, step economy, and treatment process The effect of simplicity and convenient preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a 1 mL reaction flask, add 550 microliters of phosphate buffer solution (pH=7), add 350 microliters of acetone, add 50 microliters of acetone solution of alfacalcidol (2.5mmol / L), add 50 microliters of acetone solution Oxidase (AaeUPO enzyme, 500 nmol / L). In the system, the concentration of hydrogen peroxide was 1.0 mmol / L.
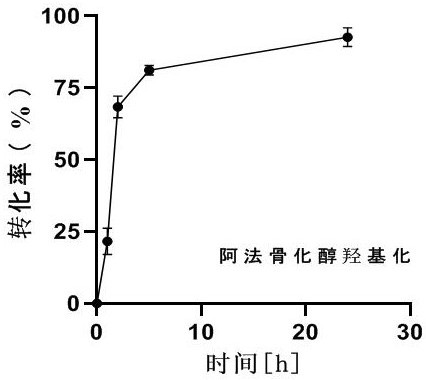
[0039] The above reaction system was reacted in a shaker at 30°C for 24 hours. After the reaction, take 100 microliters of the reaction solution, add 200 microliters of ethyl acetate, extract, dry with anhydrous sodium sulfate, and analyze by liquid chromatography. figure 1It can be seen that with the prolongation of the reaction time, the concentration of calcitriol increased, and the supernatant was collected for liquid chromatography (HPLC) to detect the content of calcitriol. HPLC detection conditions are: chromatographic column: shim-pack GIST-C18 shim-pack GIST-C18 4.6×250 mm×5 μM; mobile phase: water: acetonitrile=45:55; flow rate: 1 mL...
Embodiment 2
[0049] In a 1 mL reaction flask, add 550 microliters of phosphate buffer solution (pH=7), add 350 microliters of acetone, add 50 microliters of acetone solution of alfacalcidol (5 mmol / L), and add 75 microliters of peroxide Enzyme (MroUPO enzyme, 500 nmol / L). In the system, the concentration of hydrogen peroxide was 1.0 mmol / L at 40°C.
[0050] After the above reaction system was reacted in a shaking table at 40°C for 36 hours, the reaction was terminated, 100 μl of the reaction solution was taken, 200 μl of ethyl acetate was added, extracted, dried over anhydrous sodium sulfate, and analyzed by liquid chromatography. The yield was 55%. .
[0051] Preparation of MroUPO enzyme
[0052] Recombinant protein expression: The MroUPO nucleic acid sequence (SEQ ID NO. 9) was subcloned into the pPICZαA vector with a His-tag at the C-terminus. The pPICZαA-MroUPO recombinant plasmid was transformed into P. pastoris strain X-33, and 100 μL of competent cells were added to the linearize...
Embodiment 3
[0058] In a 1mL reaction flask, add 550 microliters of phosphate buffer solution (pH=8), add 350 microliters of acetone, add 50 microliters of acetone solution of alfacalcidol (7.5mmol / L), add 20 microliters of acetone solution Oxidase (MfeUPO enzyme, 500 nmol / L). In the system, the concentration of hydrogen peroxide was 1.0 mmol / L at 30°C.
[0059] After the above reaction system was reacted in a shaking table at 30°C for 16 hours, the reaction was terminated, 100 μl of the reaction solution was taken, 200 μl of ethyl acetate was added, extracted, dried over anhydrous sodium sulfate, and analyzed by liquid chromatography. The yield was 75%. .
[0060] Preparation of MfeUPO enzyme:
[0061] Recombinant protein expression: The MfeUPO nucleic acid sequence (SEQ ID NO. 5) was subcloned into pPICZαA vector with a His-tag at the C-terminus. The pPICZαA-MfeUPO recombinant plasmid was transformed into P. pastoris strain X-33, and 100 μL of competent cells were added to the lineari...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com