Polyoxygroup-containing boron trifluoride salt electrolyte and preparation method and application thereof
A technology of oxyboron trifluoride and electrolyte, which is applied in the field of batteries, can solve the problems of high time cost and economic cost, unpredictable results, and many uncontrollable factors, and achieve excellent electrochemical performance, improved electrochemical performance, The effect of widening the electrochemical window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Raw materials M1
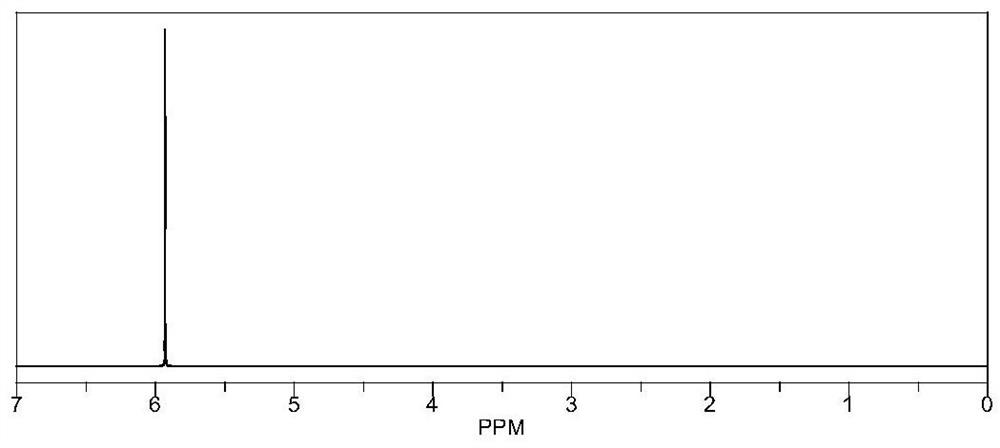
[0108] Preparation method: under nitrogen atmosphere, mix 0.01 mol of raw material and 0.04 mol of boron trifluoride tetrahydrofuran complex in 20 ml of ethylene glycol dimethyl ether, and react at room temperature for 12 hours. The obtained mixed solution was dried under reduced pressure under the conditions of 40° C. and a vacuum degree of about -0.1 MPa to remove the solvent to obtain an intermediate. Dissolve 0.04mol of lithium ethoxide in 10ml of ethanol and slowly add it to the intermediate, stir and react at 45°C for 8 hours, and dry the resulting mixed solution under reduced pressure at 45°C and a vacuum degree of about -0.1MPa, and the obtained solid is N-butyl ether was washed three times, filtered and dried to obtain product M1, wherein Q is OBF 3 Li. The yield was 85%, NMR as figure 1 shown.
Embodiment 2
[0109] Example 2: Raw materials M2
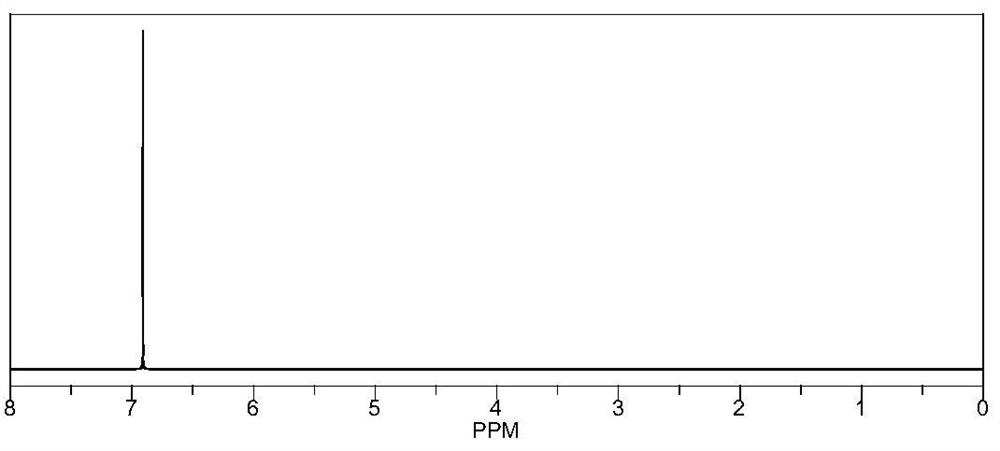
[0110] Preparation method: under argon atmosphere, slowly add 0.04mol metal lithium sheet to 0.01mol raw material, react at room temperature for 1 hour, then heat up to 50°C until the lithium sheet reacts completely to obtain an intermediate. 0.04mol of boron trifluoride butyl ether complex was added to the intermediate, and the reaction was stirred at 50°C for 7 hours, and the obtained mixed solution was dried under reduced pressure at 50°C and a vacuum degree of about -0.1MPa, and the obtained solid was Isopropyl ether was washed three times, filtered and dried to obtain product M2, wherein Q is OBF 3 Li. The yield was 89%, NMR as figure 2 shown.
Embodiment 3
[0111] Example 3: Raw materials M3
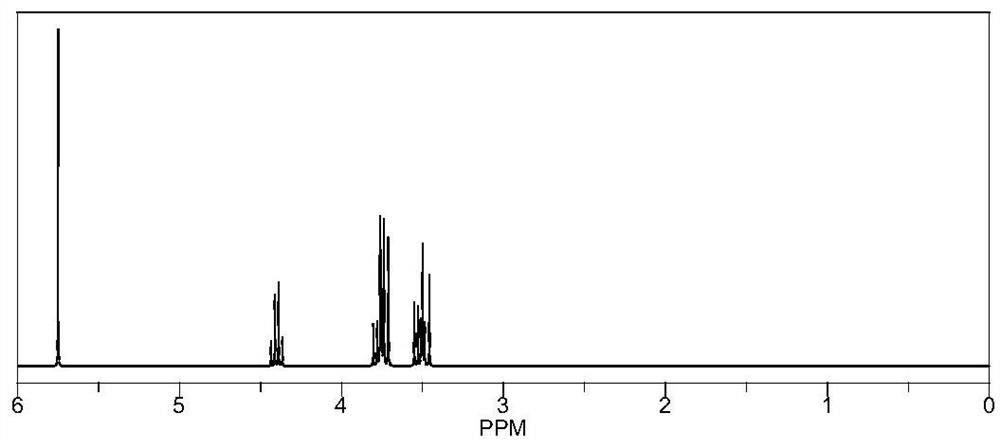
[0112] Preparation method: under a nitrogen atmosphere, 0.01 mol of raw materials and 0.05 mol of lithium hydroxide are mixed uniformly with 10 ml of methanol solution, and the reaction is carried out at room temperature for 8 hours. The obtained mixed solution was dried under reduced pressure under the conditions of 40° C. and a vacuum degree of about -0.1 MPa to remove the solvent to obtain an intermediate. 0.05mol of boron trifluoride phosphoric acid complex and 15ml of THF were added to the intermediate, and the reaction was stirred at 45°C for 12 hours, and the obtained mixed solution was dried under reduced pressure at 60°C and a vacuum degree of about -0.1MPa to obtain the obtained The crude product is filtered and dried with dichloromethane to obtain product M3, wherein Q is OBF 3 Li. The yield was 82%, NMR as image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com