Buprenorphine transdermal solution as well as preparation method and application thereof
A solution and transdermal technology, applied in the field of medicine, can solve the problems of short duration of drug effect, low bioavailability of buprenorphine, frequent administration, etc., achieve prolonged analgesic effect, avoid peak and valley phenomena, and reduce individual difference effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0053] Experimental Example 1 Preparation of buprenorphine transdermal solution
[0054] (1) Take DSPE-mPEG, disperse it in a non-polar solvent, ultrasonicate for 300w, 5min, and prepare a series of non-polar solvent solutions containing different concentrations of DSPE-mPEG;
[0055] (2) In the above solution, add buprenorphine (fixed dose of 2%, w / w) and preservatives (fixed dose of 0.05%, w / w), dissolve by ultrasonic until the solution is clear and transparent, and the ultrasonic condition is 400w, 5min.
[0056] The components and mass ratios in each recipe are set as shown in Table 1:
[0057]
Embodiment 2
[0058] Example 2 Study on the physicochemical properties of buprenorphine transdermal solution
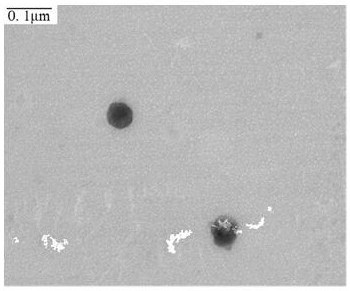
[0059] After diluting the buprenorphine transdermal solution prepared by the recipe in Example 1 by an appropriate multiple, analyze it with a particle size analyzer to obtain the particle diameter and zeta potential of the reverse micelle (three samples are measured in parallel for each recipe, and the average value is obtained. ); the prepared buprenorphine transdermal solution was moderately diluted, then added dropwise to a copper mesh covered with carbon film, and the morphology of reverse micelle particles was observed under a transmission electron microscope after treatment. The particle size analysis results of different formulations are shown in Table 2.
[0060]
[0061] In Table 2, the PDI (Polymer dispersity index) is the polymer dispersity index, which is used to describe the molecular weight distribution of the polymer.
[0062] As can be seen from Table 2, compar...
Embodiment 3
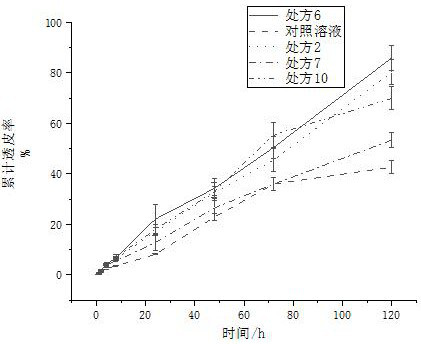
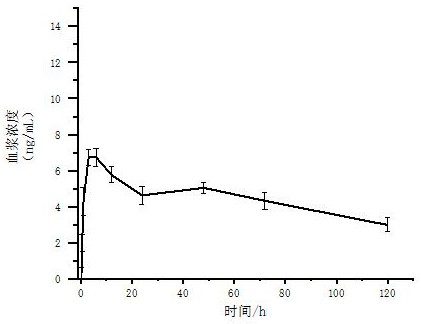
[0065] Example 3 In vitro transdermal properties of buprenorphine transdermal solution
[0066] Preparation of buprenorphine solution-IPM solution: Dissolve the prescribed amount of buprenorphine and isooctyl salicylate in IPM to prepare a clear and transparent solution as a control solution.
[0067] In vitro transdermal properties studies of buprenorphine transdermal solutions using a modified Franz diffusion cell. The skin used was the dehaired back skin of healthy adult male guinea pigs, and normal saline was used as the receiving fluid. The treated skin was placed between the sample chamber and the receiving chamber, and 2 mL of the buprenorphine-IPM solution and prescription 2, 6, 7, and 10 transdermal solutions were accurately measured and injected into the sample chamber, and then the diffusion cell was placed in the sample chamber. It was placed in a constant temperature bath (37±0.5°C) and magnetically stirred at 100r / min. Samples were taken at 0.5h, 1h, 2h, 4h, 8h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com