Novel pyrazine structure FXR agonist, preparation method and application
A compound, selected technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of low activation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
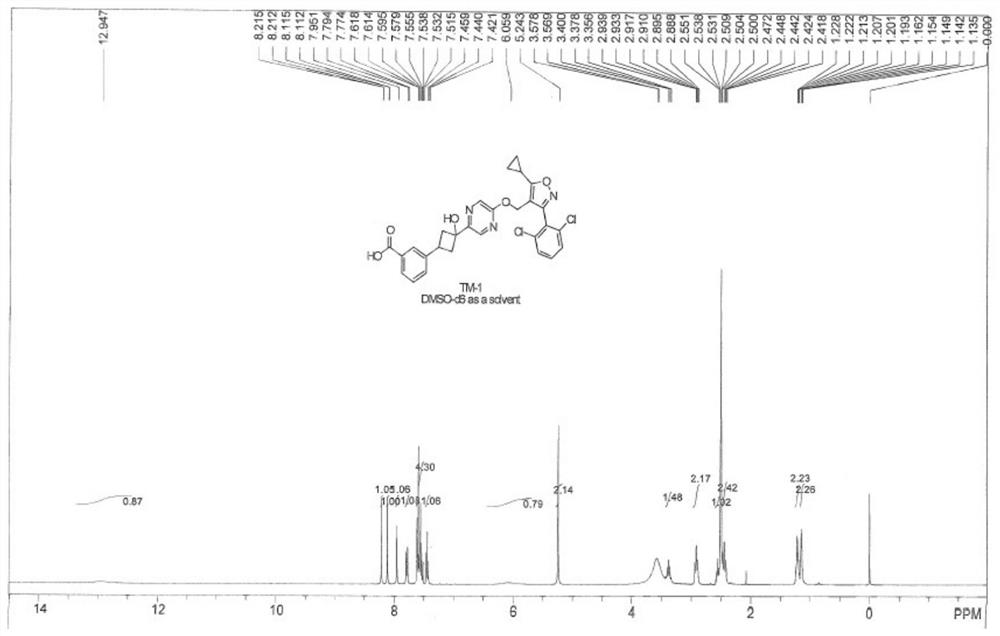
Embodiment 1
[0219] Example 1: Synthetic route of compound TM-1
[0220]
[0221] experiment procedure:
[0222] 3-(3-Bromophenyl)cyclobutanone
[0223]
[0224] To a solution of N,N-dimethylformamide (2.1 g, 24.6 mmol) in 1,2-dichloroethane (40 mL) at -15°C, trifluoromethanesulfonic anhydride (11.6 mmol) was slowly added dropwise. g, 41.0 mmol) and stirred at -15°C for 30 minutes. Then 3-bromostyrene (3.0 g, 16.4 mmol) and 2,4,6-collidine (2.9 g, 24.6 mmol) were added and stirred at room temperature overnight. The reaction was quenched by adding water, stirred at room temperature overnight, dichloromethane was added to dilute and separate the organic phase, the organic phase was washed with water and saturated brine (200 ml) respectively, dried over anhydrous magnesium sulfate, filtered with suction, concentrated under reduced pressure, and subjected to a gradient of silica gel column chromatography. Elution separation and purification (PE:EA=15:1, v / v) gave 1.3 g of 3-(3-bromoph...
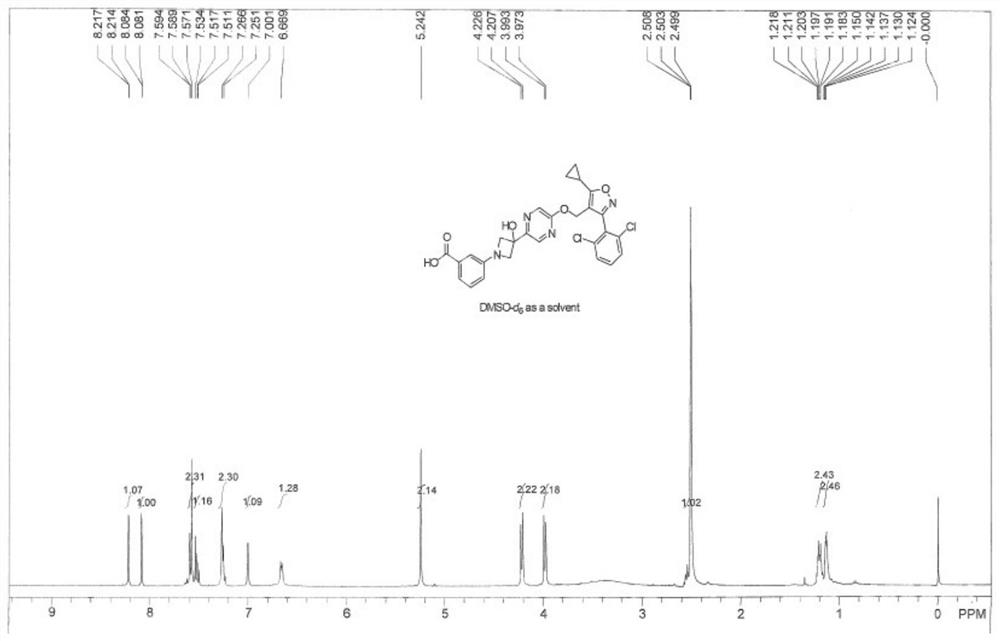
Embodiment 2
[0239] Example 2: Synthetic route of compound TM-2
[0240]
[0241] Synthesis steps:
[0242] Synthesis of methyl 3-(3-hydroxyazetidine-1-yl)benzoate 3b
[0243]
[0244] To methyl 3-iodobenzoate 3a (5.0 g, 19.1 mmol) in DMSO-D 6 (70 mL) solution was added 3-azetidin-3-ol hydrochloride (2.5 g. 22.9 mmol), Cs 2 CO 3 (15.5 g, 47.7 mmol), CuI (726 mg, 3.8 mmol) and L-proline (878 mg, 7.6 mmol), then the mixture was heated at 90 °C for 18 hours under an argon atmosphere. The solution was diluted with ethyl acetate and water, and then the organic layer was washed three times with brine, concentrated under reduced pressure, and separated and purified by silica gel column chromatography (DCM / MeOH=10 / 1, v / v) to obtain the product 3b as a white solid ( 2.7g, 68%).
[0245] Synthesis of methyl 3-(3-oxoazetidin-1-yl)benzoate 3
[0246]
[0247]Dimethyl sulfoxide (1.6 g, 20.3 mmol) was dissolved in dichloromethane (30 mL), at -78 °C, oxalyl chloride (1.3 g, 10.1 mmol) was ...
Embodiment 3
[0256] Example 3: Synthetic route of compound TM-3
[0257] 3-(3-Bromophenyl)-1-(5-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)pyrazine- 2-yl)cyclobutanol
[0258]
[0259] A 100 mL three-necked flask was protected with nitrogen, and 4-(5-bromopyrazine-2-methyleneoxy)-5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole (1.14 g, 2.3mmol) was dissolved in anhydrous tetrahydrofuran (5mL) and poured into a reaction flask, then ethanol and liquid nitrogen were added to a 500mL low-temperature Dewar flask to make the temperature drop to -78°C, and n-butyllithium (1.7ml, 2.7mmol) was slowly added dropwise. , after stirring for 10 min, slowly add 3-(3-oxocyclobutanone) methyl 3-(3-oxocyclobutanone) benzoate solution (0.56 g, 2.5 mmol) dissolved in tetrahydrofuran (10 mL) dropwise, react at -78 °C for 2 h and then warm to room temperature for reaction overnight. After the reaction was completed, the reaction solution was slowly poured into an ice-water mixture, extracte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com