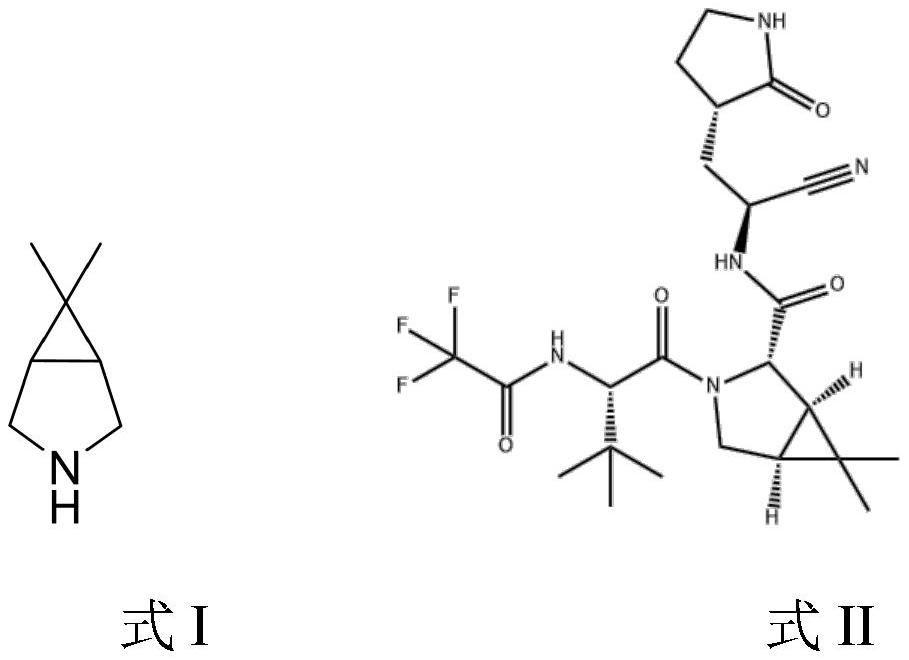
Synthesis process of intermediate bicyclic imine of novel coronavirus pneumonia resisting drug Paxlovid
A bicyclic imine and synthesis method technology, applied in the field of intermediates of the drug named Paxlovid, can solve the problems of safety risks, unsuitability for large-scale industrial production, poor yield, etc., and achieve easy availability of raw materials, significant market value and innovation Sexuality and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a solution of dichloroacetamide (1.0mol) and isopentenyl chloride (1.1mol) in THF (1L) at a temperature between 0 and 10 degrees, add 1.05 times the molar amount of NaH (60%, dispersed in mineral oil) in batches. ), continue to stir the reaction for 2 hours after the addition, and detect that one of the raw materials disappears, and the saturated aqueous ammonium chloride solution is added dropwise to neutralize the reaction. After extraction with ethyl acetate, the organic phase was concentrated to obtain compound C.
[0024] Dissolve C with THF, add substrate 0.05 mol of cobalt chloride, 0.1 mol of ligand L2 and 1.2 mol of zinc powder, heat to 50-60 degrees and react for 3 hours until the substrate disappears, and then concentrate to dryness to obtain compound D.
[0025] Compound D was dissolved in water and isopropanol, then 2.0 moles of sodium borohydride and 2.0 moles of trimethylchlorosilane were added simultaneously in batches until the reaction was complete,...
Embodiment 2
[0028] In the solution DMF (1L) of dichloroacetamide (1.0mol) and isopentenyl bromide (0.9mol) at room temperature, 1.5mol potassium carbonate was added in batches, and the stirring reaction was continued for 5 hours after the addition. The raw materials disappeared, the solid was removed by filtration, 3 L of ethyl acetate and 2 L of water were added to the mother liquor, stirred, stood, separated, extracted, and the organic phase was concentrated to obtain compound C.
[0029] Dissolve C with dioxane, add substrate 0.05 mol of cobalt bromide, 0.1 mol of zinc bromide, 0.1 mol of ligand L2 and 1.5 mol of zinc powder, heat to 80-90 degrees and react for 2 hours until the substrate disappears, It was then concentrated to dryness to give compound D.
[0030] Compound D was dissolved in THF, and then 1.5 mol of red aluminum solution was added in batches at the same time until the reaction was complete. After quenching with hydrochloric acid, most of the alcohol was evaporated by c...
Embodiment 3
[0033] In the toluene (1 L) solution of dichloroacetamide (1.0 mol) and isopentenyl bromide (0.9 mol) at room temperature, 1.1 mol of potassium tert-butoxide was added in batches, and after the addition, the reaction was continued for 1 hour, and water ( 1L) quenched, stirred, stood, layered, extracted, and the organic phase was concentrated to obtain compound C.
[0034] Dissolve C with methyl tert-butyl ether, add 0.05 mol of nickel chloride, 0.1 mol of ligand L1 and 1.0 mol of zinc powder as the substrate, heat to 40-50 degrees and react for 2 hours until the substrate disappears to obtain compound D. THF solution.
[0035] To the THF solution of the above compound D, 1.2 moles of tetrahydroaluminum lithium were added in batches until the reaction was complete. After quenching with hydrochloric acid, most of the THF was evaporated under reduced pressure, extracted with dichloromethane, and the organic phase was concentrated under normal pressure. The residue was distilled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com