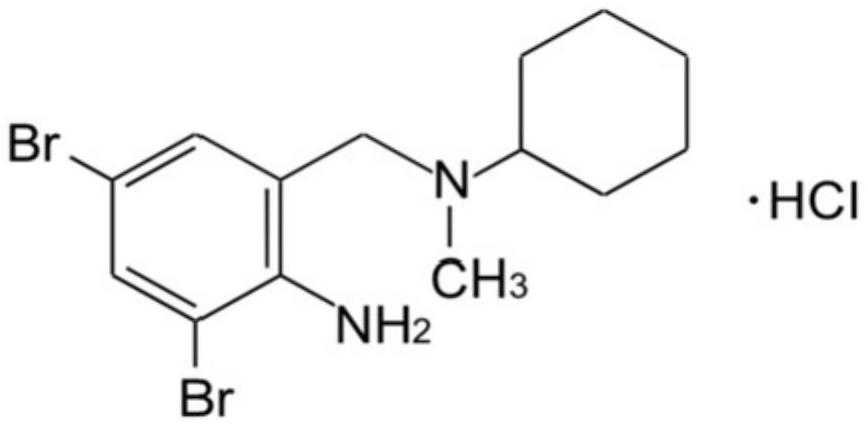
Stable bromhexine hydrochloride solution for inhalation and preparation method thereof
A bromhexine hydrochloride solution technology, applied in the field of medicine, can solve problems such as large amount of excipients and drug safety issues, and achieve the effect of ensuring drug safety and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
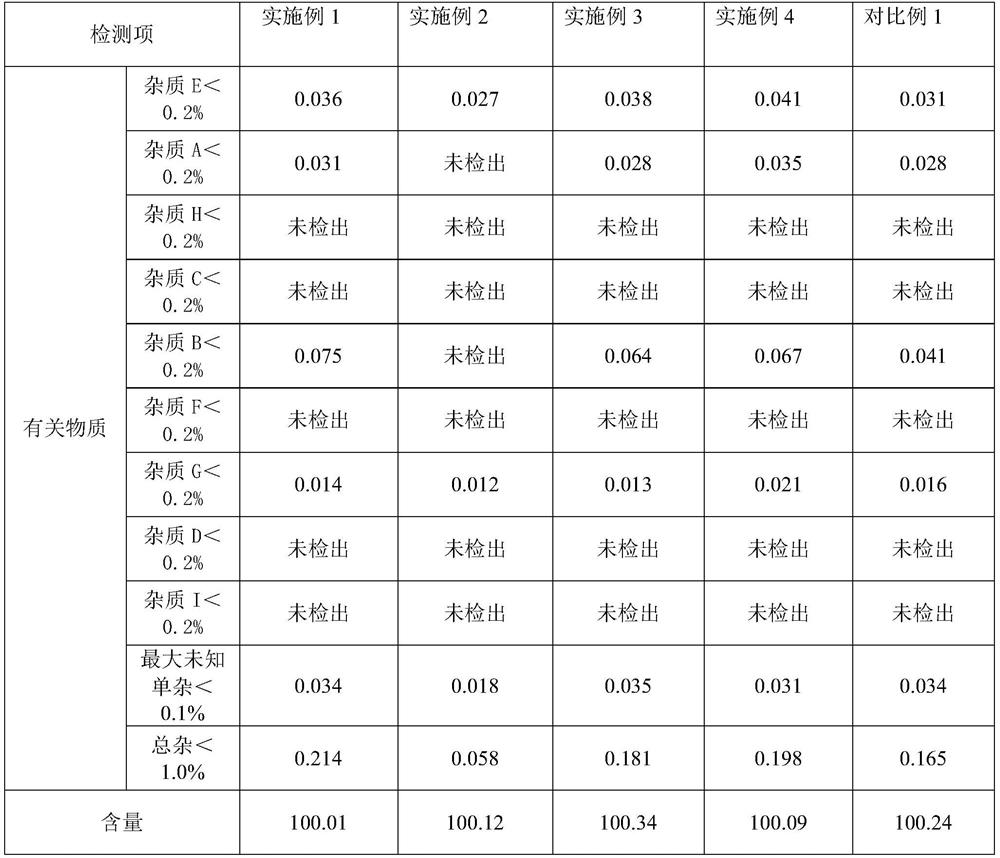
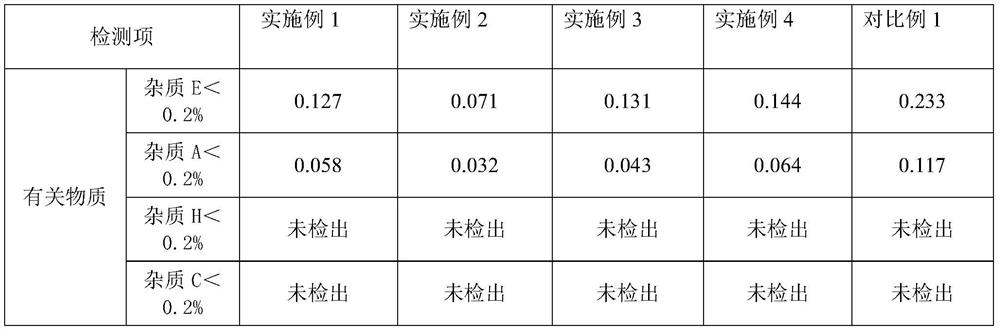
Examples
Embodiment 1
[0041] prescription:
[0042] Bromhexine hydrochloride 2g
[0043] DL-tartaric acid 1g
[0044] L-serine 0.1g,
[0045] Water for injection was added to 1000mL,
[0046] Preparation:
[0047] 1) grinding after mixing bromhexine hydrochloride and L-serine to obtain the mixture of bromhexine hydrochloride and L-serine;
[0048] 2) Take 90% water for injection, add DL-tartaric acid under stirring, and after dissolving, add the mixture of bromhexine hydrochloride and L-serine and stir until completely dissolved, adjust pH to 3 with sodium hydroxide, and add water for injection to the full amount;
[0049] 3) Filtration and filling:
[0050] After constant volume, the liquid medicine is stirred evenly, filtered through a 0.22 μm polyethersulfone filter membrane, filled, filled with nitrogen, and melted and sealed.
Embodiment 2
[0052] Bromhexine hydrochloride 2g
[0053] DL-tartaric acid 1g
[0054] L-serine 0.2g,
[0055] Water for injection was added to 1000mL,
[0056] Preparation:
[0057] 1) grinding after mixing bromhexine hydrochloride and L-serine to obtain the mixture of bromhexine hydrochloride and L-serine;
[0058] 2) Take 90% water for injection, add DL-tartaric acid under stirring, and after dissolving, add the mixture of bromhexine hydrochloride and L-serine and stir until completely dissolved, adjust pH to 3 with sodium hydroxide, and add water for injection to the full amount;
[0059] 3) Filtration and filling:
[0060] After constant volume, the liquid medicine is stirred evenly, filtered through a 0.22 μm polyethersulfone filter membrane, filled, filled with nitrogen, and melted and sealed.
Embodiment 3
[0062] Bromhexine hydrochloride 2g
[0063] DL-tartaric acid 1g
[0064] L-serine 0.3g,
[0065] Water for injection was added to 1000mL,
[0066] Preparation:
[0067] 1) grinding after mixing bromhexine hydrochloride and L-serine to obtain the mixture of bromhexine hydrochloride and L-serine;
[0068] 2) Take 90% water for injection, add DL-tartaric acid under stirring, and after dissolving, add the mixture of bromhexine hydrochloride and L-serine and stir until completely dissolved, adjust pH to 3 with sodium hydroxide, and add water for injection to the full amount;
[0069] 3) Filtration and filling:
[0070] After constant volume, the liquid medicine is stirred evenly, filtered through a 0.22 μm polyethersulfone filter membrane, filled, filled with nitrogen, and melted and sealed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com