Carbonyl reductase and method for preparing ethyl (R)-4-chloro-3-hydroxybutyrate by using carbonyl reductase
A technology of carbonyl reductase and reductase, which is applied in the field of bioengineering, can solve the problems of expensive metal catalysts, harsh reactor requirements, and harsh reaction conditions, so as to improve biotransformation efficiency, increase reaction substrate concentration, and stereoselectivity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Construction and culture of genetically engineered bacteria E.coli BL21(DE3) / pET28a-CR1
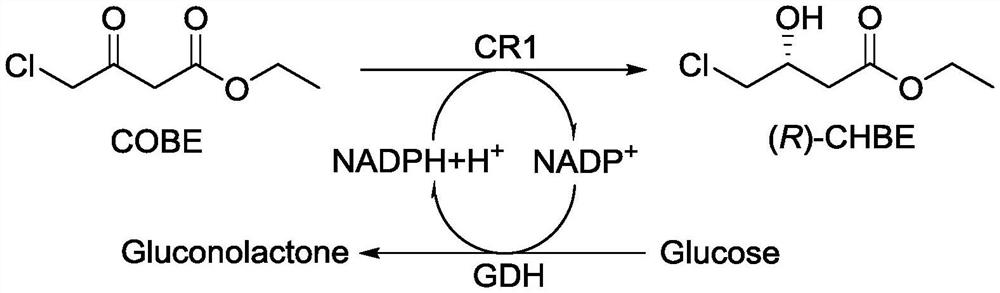
[0022] The gene sequence of carbonyl reductase CR1 as shown in SEQ ID NO.2 was cloned into pET28a plasmid, and the restriction sites were NdeI and EcoRI to obtain recombinant plasmid pET28a-CR1, and then recombinant plasmid pET28a-CR1 was imported into Escherichia coli E. coliBL21(DE3) was constructed to obtain a genetically engineered strain E. coli BL21(DE3) / pET28a-CR1.
[0023] The engineered bacteria E.coli BL21(DE3) / pET28a-CR1 constructed above were inoculated into LB medium containing kanamycin (50 μg / mL), and cultured at 37°C at 200 rpm. When the optical density of the bacterial solution (OD600) When it reaches 0.6-0.8, add 0.1mM isopropyl-β-D-thiogalactoside (IPTG) for induction, the induction temperature is 16°C, and the induction time is 20 hours. Genetically engineered bacterial wet cells with carbonyl reductase CR1.
[0024] 2. Construction and culture of genetical...
Embodiment 2
[0033] In this example, the genetically engineered bacteria E.coli BL21(DE3) / pET28a-CR1 and the genetically engineered bacteria E.coli BL21(DE3) / pET28a-GDH obtained in Example 1 were used to synthesize (R)-CHBE, Specifically include the following steps:
[0034] 40g COBE was dissolved in 20mL n-octanol to obtain an oil phase; 2.0g GR1 engineering bacteria wet cells (E.coliBL21(DE3) / pET28a-CR1), 2.0g GDH engineering bacteria wet cells (E.coli BL21(DE3) ) / pET28a-GDH), 3.0 g glucose and 0.03 g NADPH were added to 90 mL of 0.1 mol / L KH, pH=7.5 2 PO 4 -K 2 HPO 4 In the buffer solution, the aqueous phase is obtained; the oil phase and the aqueous phase are mixed, the pH value is adjusted to about 7.0, and the reaction is stirred at room temperature for 3 hours. After the reaction is completed, it is extracted three times with an equal volume of ethyl acetate. The organic solvent was distilled off under reduced pressure to obtain 37.9 g of (R)-CHBE (ee value>99.1%, yield 95%, con...
Embodiment 3
[0036]In this example, the genetically engineered bacteria E.coli BL21(DE3) / pET28a-CR1 and the genetically engineered bacteria E.coli BL21(DE3) / pET28a-GDH obtained in Example 1 were used to synthesize (R)-CHBE, Specifically include the following steps:
[0037] Dissolve 400g COBE in 50mL n-octanol to obtain an oil phase; 5.0g GR1 engineering bacteria wet cells (E.coliBL21(DE3) / pET28a-CR1), 5.0g GDH engineering bacteria wet cells (E.coli BL21(DE3) ) / pET28a-GDH), 7.5g glucose and 0.3g NADPH were added to 950mL KH of 0.1mol / L, pH=7.5 2 PO 4 -K 2 HPO 4 In the buffer solution, the water phase was obtained; the oil phase and the water phase were mixed, the pH value was adjusted to about 7.0, and the reaction was stirred at room temperature for 2 h. After the reaction was completed, it was extracted three times with an equal volume of ethyl acetate. The organic solvent was distilled off under reduced pressure to obtain 388.2 g of (R)-CHBE (ee value>99.5%, yield 97%, conversion 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com