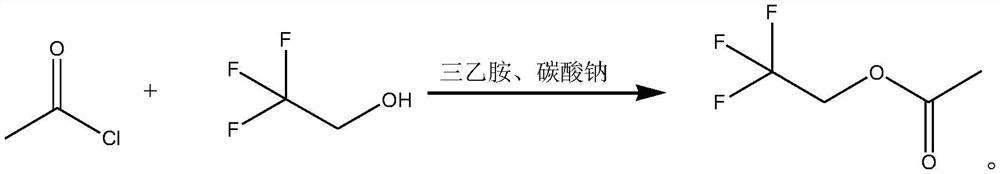
Synthesis method of trifluoroethanol acetate
A technology of trifluoroethanol acetate and its synthesis method, which is applied to the preparation of carboxylic acid halides, the manufacture of final products, organic chemistry, etc., and can solve the problems of poor purity, imperfect manufacturing and purification methods of trifluoroethanol acetate, and low production capacity, etc. problems, to achieve the effects of improving yield and purity, inhibiting side reactions, and simplifying the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis method of trifluoroethanol acetate, the synthesis steps of which are as follows:
[0021] Take 86.35g (1.1mol) acetyl chloride dissolved in 200mL of dichloromethane to give dichloromethane solution of acetyl chloride;
[0022]Take 100g (1mol) of trifluoroethanol to cool down to 0 °C, maintain 0 °C slowly add dichloromethane solution of acetyl chloride (drop time is 1h), after the drop is completed, 0 °C stirring reaction for 1h, and then add 183.98g (1.82 mol) of triethylamine in 4 batches (triethylamine is generally added in 3 to 4 batches), two drops of triethylamine are stirred between the acylation reaction for 1h, triethylamine drops are completed, and then maintain the 0 °C reaction for 1h, add 19.27g (0.18mol) Sodium carbonate, continue to maintain 0 °C reaction for 1h, then heated to 5 °C reaction 1h, and then heated to 10 °C reaction to trifluoroethanol all reaction completely, and then add 200mL of water washing, phase separation, the resulting organic p...
Embodiment 2
[0025] Synthesis method of trifluoroethanol acetate, the synthesis steps of which are as follows
[0026] Take 78.5 g (1mol) of acetyl chloride dissolved in 180mL of dichloromethane to give dichloromethane solution of acetyl chloride;
[0027] Take 100g (1mol) of trifluoroethanol to cool down to -5 °C, maintain -5 °C slowly add dichloromethane solution of acetyl chloride (drop time is 1h), after the drop is completed, -5 °C stirring reaction 0.5h, and then add 160.22g (1.58 mol) of triethylamine in 3 batches (triethylamine is generally added in 3 to 4 batches), the two drops of triethylamine are stirred to carry out the acylation reaction for 0.5h, after the triethylamine drops are completed, then maintain the -5 °C reaction 0.5h, add 33.57g (0.32mol) sodium carbonate, continue to maintain -5 °C reaction 0.5h, then heat up to 8 °C reaction 0.5h, and then heat up to 15 °C reaction to trifluoroethanol all reaction completely, and then add 200mL of water washing, phase separation, th...
Embodiment 3
[0029] Synthesis method of trifluoroethanol acetate, the synthesis steps of which are as follows:
[0030] Take 82.43g (1.05mol) acetyl chloride dissolved in 190mL of dichloromethane to give dichloromethane solution of acetyl chloride;
[0031]Take 100g (1mol) of trifluoroethanol to cool down to -1 °C, maintain -1 °C slowly add acetyl chloride dichloromethane solution (drop time is 1h), after the drop is completed, -1 °C stirring reaction 0.6h, and then add 162.78g (1.61 mol) of triethylamine in 3 batches (triethylamine is generally added in 3 to 4 batches), the two drops of triethylamine are stirred to carry out the acylation reaction for 0.6h, after the completion of the triethylamine drop,then maintain the -1 °C reaction 0.6h, add 25.58g (0.24mol) sodium carbonate, continue to maintain -1 °C reaction 0.6h, then heated to 7 °C reaction 0.6h, and then heated to 12 °C reaction to trifluoroethanol all reaction completely, and then added 200mL of water washing, phase separation, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com