Oxygen-mediated free radical polymerization method
A free radical-mediated technology, applied in the field of polymers, to achieve good catalytic performance, low energy consumption, and strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
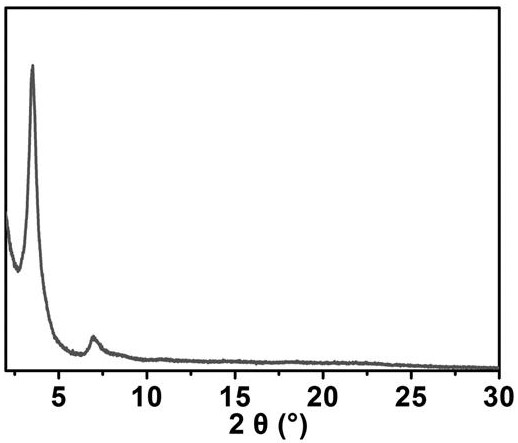
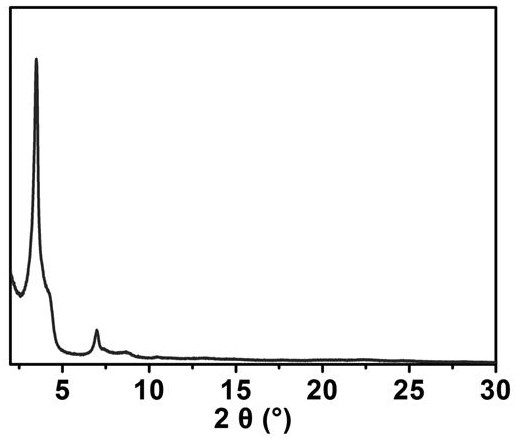
[0041] (1) 4,4',4'',4'''-(porphyrin-5,10,15,20-tetra)aniline (H 2 -TCPP) synthesis: Add 4-nitrobenzaldehyde (22.0 g, 145 mmol), propionic acid (600 mL) and acetic anhydride (24 mL) into a 1000-mL flask under N 2 middle reflux. Subsequently, pyrrole (10 mL) was added via syringe, and the mixture was reacted for 12 h. After the mixture was cooled to room temperature, it was placed in the refrigerator overnight, and the solid was filtered, washed with distilled water (200 mL) and methanol (500 mL), and dried in vacuo. Subsequently, the solid was dissolved in pyridine (160 mL) with N 2 Refluxed for 12 h under protection, the mixture was cooled to room temperature, filtered to obtain a black solid, washed with acetone to obtain a purple solid. The product and SnCl 2 2H 2 O (18.0 g, 7.97 mmol) was added to hot concentrated hydrochloric acid (500 mL) solution, N 2 Reflux overnight under atmosphere. The mixture was cooled to 0 °C with ice water and neutralized to pH = 8 with co...
Embodiment 2
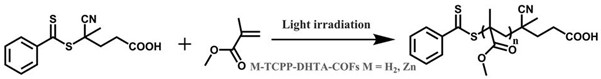
[0048] Get embodiment 1 product (1) H 2 -TCPP-DHTA-COFs 1 mg, MMA (2 mL, 18.6 mmol), CPADB (26 mg, 0.093 mmol), TEA (134 μL, 0.93 mmol) and DMAC (1 mL) were added to a 4 mL glass bottle, and the stopper was closed Rubber stopper, with white LED strip (13W m -1 , 15mW cm -2 ) irradiation. Take a small amount of the mixture at a pre-designed time, and use gel permeation chromatography (GPC) to measure the conversion rate and molecular weight M n and molecular weight distribution M w / M n and other parameters.
[0049] image 3 A schematic diagram of the polymerization process; Figure 4 is the molecular weight M n ,The molecular weight distribution M w / M n Relational figure with conversion rate and the outflow curve figure of the polymer product measured by GPC; Figure 5 for ln( M 0 / M t ) Polymerization kinetics curve as a function of time t, where M 0 is the initial monomer concentration, M t is the monomer concentration at time t, it can be seen ...
Embodiment 3
[0052] Take the product of Example 1 (2) Zn-TCPP-DHTA-COFs 1 mg, MMA (2 mL, 18.6 mmol), CPADB (26 mg, 0.093 mmol), TEA (134 μL, 0.93 mmol) and DMAC (1 mL) were added 4 mL glass vial with a rubber stopper and a white LED strip (13W m -1 , 15mW cm -2 ) irradiation. Take a small amount of the mixture at a pre-designed time, and use gel permeation chromatography (GPC) to measure the conversion rate and molecular weight M n and molecular weight distribution M w / M n and other parameters.
[0053] Image 6 is the molecular weight M n ,The molecular weight distribution M w / M n Graph vs. conversion rate, Figure 7 It is a polymerization kinetic curve, and the combination of the two shows that a polymer with controllable molecular weight and narrow molecular weight distribution can be obtained under this polymerization condition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com