Method for detecting genotoxic impurities in indobufen bulk drug
A genotoxicity and detection method technology, applied in the direction of measuring devices, material separation, analysis of materials, etc., can solve the problems of difficult detection and low limit, and achieve the effects of high sensitivity, strong specificity, and simple experimental operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
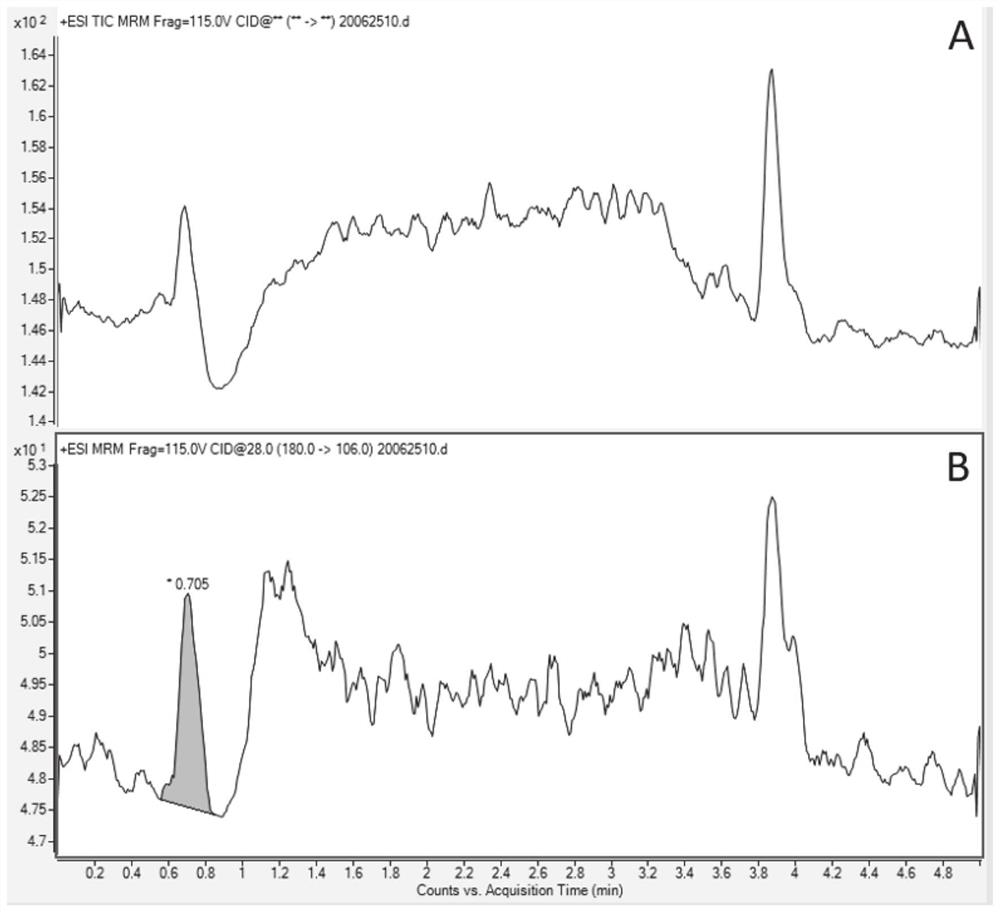
[0059] Example 1 Separation and determination of genotoxic impurity 2-(4-aminophenyl) butyric acid in indobufen raw materials by liquid chromatography-mass chromatography
[0060] (1) Chromatographic conditions
[0061] Instrument: Agilent 6470 HPLC triple quadrupole mass spectrometer
[0062] Chromatographic column: ACQUITY BEH C18 2.1mm×50mm, 1.7μm
[0063] Mobile phase: Solvent A: 0.01% acetic acid aqueous solution; Solvent B: acetonitrile, eluted according to the following gradient:
[0064]
[0065] Diluent: Acetonitrile-Water (50:50)
[0066] Flow rate: 0.3mL / min
[0067] Column temperature: 35°C
[0068] Injection volume: 5μl
[0069] Mass spectrometry conditions: Electrospray ion source, positive ion scan mode
[0070] The drying gas, atomizing gas and sheath gas are all nitrogen; the collision gas is high-purity nitrogen;
[0071] Drying gas temperature: 300°C
[0072] The drying gas flow rate is: 6L / min
[0073] Atomizing gas pressure: 35psi
[0074] She...
Embodiment 2
[0102] (1) Except for the chromatographic column and elution gradient, other chromatographic conditions are the same as in Example 1. The chromatographic column is ZORBAX SB-C8 3.0×100mm, 1.8 μm, and the elution gradient is:
[0103] time (min) 0.01 1 3 3.1 5 Solvent A(%) 90 90 10 90 90 Solvent B(%) 10 10 90 10 10
[0104] (2) solution preparation and assay method are all with embodiment 1
[0105] (3) Sample injection and result analysis
[0106] Take diluent blank, reference substance solution (concentration is 30% of limit, 1.125ng / mL), standard-added need testing solution sample injection respectively, record chromatogram. Results The peaks of 2-(4-aminophenyl)butyric acid could achieve baseline separation under these conditions, and there was no interference from the blank solution, samples and impurities in the samples; the S / N was 16.9, greater than 10. The specificity and sensitivity of the method can meet the requirements for t...
Embodiment 3
[0115] Example 3 Separation and determination of genotoxic impurity 2-(4-nitrophenyl)butyric acid in indobufen raw materials by liquid chromatography-mass chromatography
[0116] (1) Chromatographic conditions
[0117] Instrument: Agilent 6470 HPLC triple quadrupole mass spectrometer
[0118] Chromatographic column: ACQUITY BEH C18 2.1mm×50mm, 1.7μm
[0119] Mobile phase: solvent A: 20mmol / L ammonium acetate solution; solvent B: acetonitrile, eluted according to the following gradient:
[0120] time (min) 0.01 1 2 4 4.1 5 Solvent A(%) 90 90 10 10 90 90 Solvent B(%) 10 10 90 90 10 10
[0122] Flow rate: 0.3mL / min
[0123] Column temperature: 35°C
[0124] Injection volume: 2μl
[0125] Mass spectrometry conditions: Electrospray ion source, negative ion scan mode
[0126] The drying gas, atomizing gas and sheath gas are all nitrogen; the collision gas is high-purity nitrogen;
[0127] Drying gas tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com