Fluorescent probe compound, preparation method and application of fluorescent probe compound as superoxide anion indicator
A technology of superoxide anion and fluorescent probe, which is applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, chemical instruments and methods, etc., can solve problems such as death and cell damage, and achieve small damage to cells and living bodies and biocompatibility Good performance, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
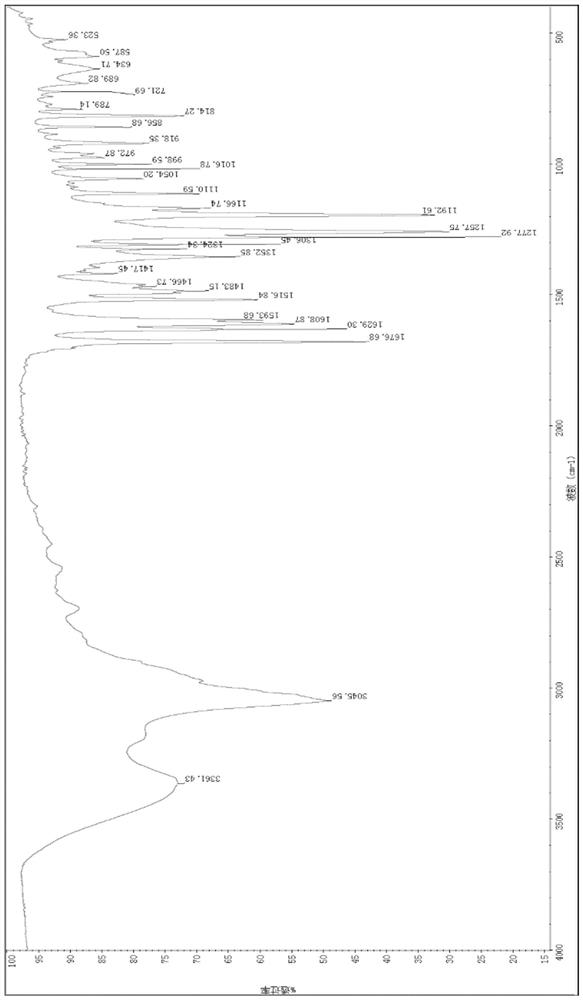
[0048] Example 1 Fluorescent Probe Cell membrane-O 2 ·- Preparation and structure elucidation
[0049] Fluorescent probe Cell membrane-O 2 ·- The preparation method comprises the steps:
[0050] (1) Dissolve the raw materials 3-bromopropyltrimethylammonium bromide (5mmol) and triethylamine (5.5mmol) in the reaction solvent 10mL N, N-dimethylformamide to activate the carboxyl group at 80°C for 15min;
[0051] (2) Add caffeic acid (5 mmol), and stir at 80° C. for 6 h;
[0052] (3) After the above reaction was completed, the solvent was removed by rotary evaporation under reduced pressure, and the heating temperature was 70°C. Use ethyl acetate:methanol=3:1 as developing solvent, carry out thin-layer chromatography chromatographic separation and purification, finally obtain light yellow fluorescent probe Cellmembrane-O 2 ·- (30%).
[0053] Mass Spectrometry Characterization:
[0054] HRMS(ESI)m / z:[M-H]calculated for C 15 h 22 NO 4 + , 280.1549found 280.1537. NMR char...
Embodiment 2
[0056] Example 2 O 2 ·- in vitro testing
[0057] Usually, the amphiphilic fluorescent probe Cell membrane-O 2 ·- Dissolve in physiological saline, PBS buffer or organic solvents such as methanol and dimethyl sulfoxide, and then add appropriate buffer and other organic reagents for testing. The probe Cell membrane-O was studied separately 2 ·- Photophysical properties and cytotoxicity in cells in a cell disruption solution simulating a physiological environment.
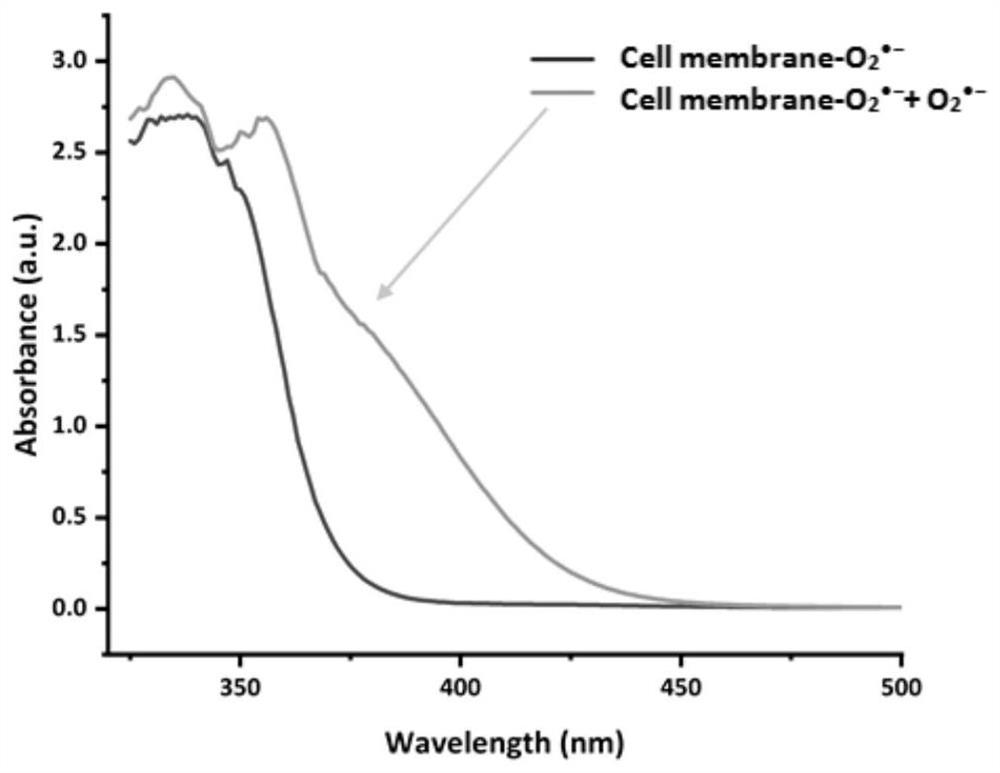
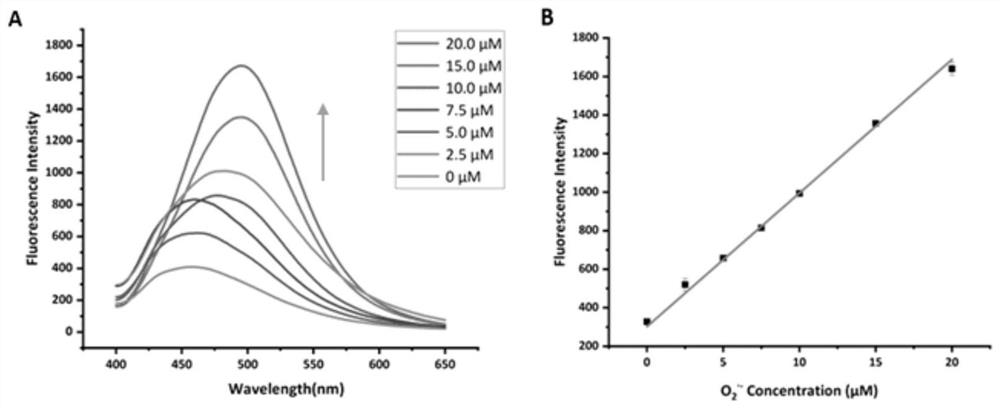
[0058] Probe Cell membrane-O 2 ·- with O 2 ·- UV absorption, fluorescence emission, reversible change, photostability and selectivity experiments of the reaction
[0059] Control group: Cell membrane-O 2 ·- (400μM), cell disruption solution, DMSO; experimental group: Cell membrane-O 2 ·- (400μM), cell disruption solution, O 2 ·- (20 μM). Add 0 and 20 μM O to the probe 2 ·- , measure its ultraviolet absorption spectrum, as the control group and the experimental group, its spectrum is shown in figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com