Cetirizine hydrochloride tablet and preparation method thereof
A technology of cetirizine hydrochloride and rizine tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of difficult control of process quality, failure to completely overcome die corrosion and Problems such as sticking, cumbersome process, etc., achieve the effect of reducing the difficulty of process quality control, simplifying production operation procedures, and reducing additives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
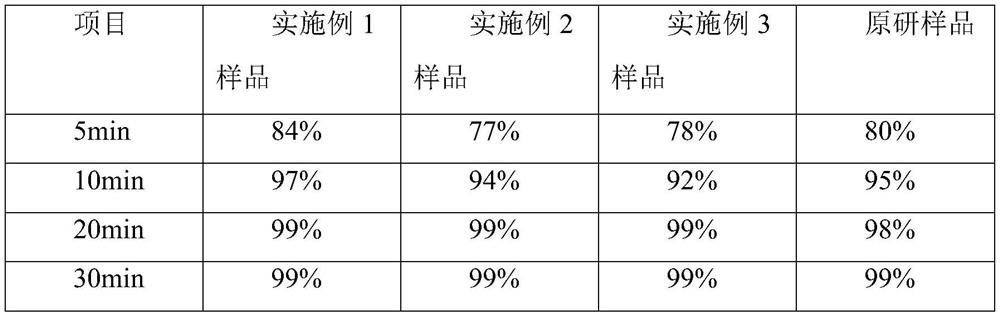
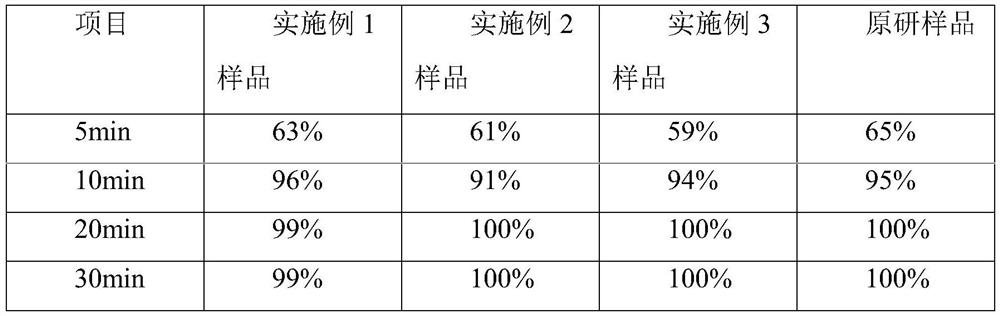
Embodiment 1
[0032] A kind of cetirizine hydrochloride tablet is prepared from following components by weight:
[0033] 10 parts of cetirizine hydrochloride, 50 parts of porous silicon, and 60 parts of microcrystalline cellulose 102.
[0034] Among them, the pore size distribution of porous silicon measured by laser particle size analyzer is 17-25 μm at D50, and the pore size distribution is 55-70 μm at D90 measured by laser particle size analyzer.
[0035] Cetirizine hydrochloride is a drug raw material, which is a off-white powder, and the content of cetirizine hydrochloride in this type of white powder reaches the Chinese Pharmacopoeia standard (greater than 99.0%). In this program, the cetirizine hydrochloride drug raw material is directly used, No preprocessing is required.
[0036] In this solution, the particle size of the microcrystalline cellulose 102 is greater than 60 mesh, and the loss on drying is less than 7.0%.
[0037] A kind of preparation method of cetirizine hydrochlor...
Embodiment 2
[0042] A kind of cetirizine hydrochloride tablet is prepared from following components by weight:
[0043] 10 parts of cetirizine hydrochloride, 40 parts of porous silicon, 40 parts of microcrystalline cellulose 112, and 30 parts of lactose.
[0044] Among them, the pore size distribution of porous silicon measured by laser particle size analyzer is 17-25 μm at D50, and the pore size distribution is 55-70 μm at D90 measured by laser particle size analyzer.
[0045] Cetirizine hydrochloride is a drug raw material, which is a off-white powder, and the content of cetirizine hydrochloride in this type of white powder reaches the Chinese Pharmacopoeia standard (greater than 99.0%). In this program, the cetirizine hydrochloride drug raw material is directly used, No preprocessing is required.
[0046] Further, the particle size of the microcrystalline cellulose 112 is greater than 60 mesh, and the loss on drying is less than 1.5%.
[0047] Further, the lactose is spray-dried lacto...
Embodiment 3
[0053] A kind of cetirizine hydrochloride tablet is prepared from following components by weight:
[0054] 10 parts of cetirizine hydrochloride, 30 parts of porous silicon, 40 parts of microcrystalline cellulose 112, and 40 parts of lactose.
[0055] Among them, the pore size distribution of porous silicon measured by laser particle size analyzer is 17-25 μm at D50, and the pore size distribution is 55-70 μm at D90 measured by laser particle size analyzer.
[0056] Cetirizine hydrochloride is a drug raw material, which is a off-white powder, and the content of cetirizine hydrochloride in this type of white powder reaches the Chinese Pharmacopoeia standard (greater than 99.0%). In this program, the cetirizine hydrochloride drug raw material is directly used, No preprocessing is required.
[0057] In this solution, the particle size of the microcrystalline cellulose 112 is greater than 60 mesh, and the loss on drying is less than 1.5%.
[0058] The lactose is spray-dried lacto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com